Compound derived from turmeric essential oil has neuroprotective properties

Researchers from Kumamoto University, Japan have found that a component derived from turmeric essential oil, aromatic turmerone (ar-turmerone), and its derivatives act directly on dopaminergic nerves to create a neuroprotective effect on tissue cultures of a Parkinson’s disease model. This appears to be due to enhanced cellular antioxidant potency from the activation of Nrf2. The researchers believe that the ar-turmerone derivatives identified in this study can be used as new therapeutic agents for Parkinson’s disease.
Parkinson’s disease is a neurodegenerative disease caused by the selective death of dopaminergic neurons that transmit information from the substantia nigra of the midbrain to the striatum which results in decreased dopamine production. Symptoms include limb tremors, immobility, muscle rigidity, and other movement disorders. Treatments, such as dopamine supplements, are currently available but there still no way to inhibit dopaminergic neurodegeneration.
Previous studies have reported that the inflammatory response caused by the activation of microglia (cells responsible for immune function in the brain) is observed in the substantia nigra of the midbrain of Parkinson’s disease patients. Further experiments designed to mimic the in vivo state of the midbrain (midbrain slice culture) revealed that microglial activation triggers the selective degeneration of dopaminergic neurons in the substantia nigra, and that nitric oxide (NO) derived from activated microglia was involved in the neurodegeneration. These findings suggest that compounds with anti-inflammatory effects on microglia may suppress dopaminergic degeneration.
Thus, researchers analyzed aromatic tumerone (ar-turmerone), a major component of turmeric essential oil that has been reported to exhibit anti-tumor and anti-inflammatory effects on microglia. They used the BV2 microglial cell line and midbrain slice cultures to 1) determine if ar-turmerone suppresses dopaminergic neurodegeneration through its anti-inflammatory effects, and 2) identify structurally similar compounds (derivatives) that might have stronger anti-inflammatory and neuroprotective effects.

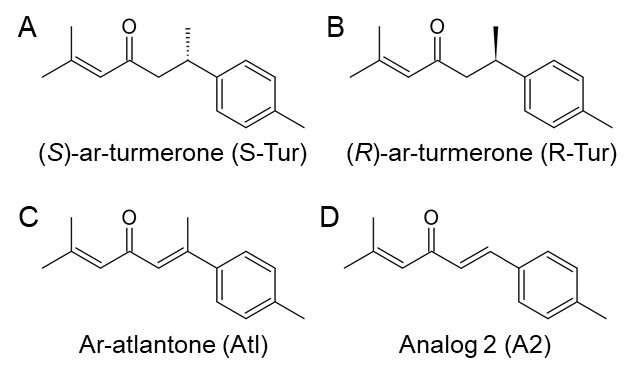
Ar-turmerone has an asymmetric carbon (S-Tur) so researchers prepared eight analogs and attempted to identify those with stronger anti-inflammatory effects. They used the inhibitory effects on the inflammatory response as induced by lipopolysaccharide (LPS)-stimulated activation of BV2 cells as an indicator. The analogs with stronger anti-inflammatory effects than S-Tur were (R)-ar-turmerone (R-Tur), ar-atlantone (Atl), and analog 2 (A2).
To examine whether these compounds, including S-Tur, have an inhibitory effect on dopaminergic degeneration, researchers then observed midbrain slice cultures in which microglial activation was induced by interferon-γ and LPS stimulation (IFN-γ/LPS). All four compounds significantly suppressed a decrease in the number of dopaminergic neurons as induced by IFN-γ/LPS. However, the production of NO, which is released from activated microglia and is involved in dopaminergic neurodegeneration, was not inhibited at all. In addition, three compounds, S-Tur, Atl and A2, inhibited dopaminergic degeneration that is induced by MPP+, a toxin that selectively damages dopaminergic neurons independent of microglial activity. These results indicate that S-Tur and its derivatives, Atl and A2, have a direct effect on dopaminergic neurons and exhibit neuroprotective effects. Furthermore, analysis using dopaminergic progenitor cell lines and midbrain slice cultures revealed that the neuroprotective effects of Atl and A2 are mediated by activation of Nrf2, a transcription factor that enhances the antioxidant potency of cells.
“Our study elucidated a new mechanism by which ar-turmerone and its derivatives directly protect mesencephalic slice dopaminergic neurons, independent of their previously reported anti-inflammatory effects on microglia,” said Associate Professor Takahiro Seki, who led the study. “We showed that two derivatives, Atl and A2, exhibit neuroprotective effects by increasing the expression of antioxidant proteins through the activation of Nrf2. In particular, the analog A2 identified in this study is a potent activator of Nrf2 and is assumed to have a strong antioxidant effect. We think it is possible that this compound may be a new dopaminergic neuroprotective agent for Parkinson’s disease treatment, and it could also be used to treat other diseases caused by oxidative stress, such as liver and kidney diseases.”
Source: Read Full Article