how to test for low testosterone at home
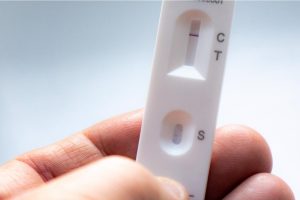
In a recent study posted to the medRxiv* preprint server, researchers evaluated clinical accuracy and analytical detection capabilities of the two US Food and Drug Administration (FDA)-approved rapid antigen tests (RATs) across severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants.
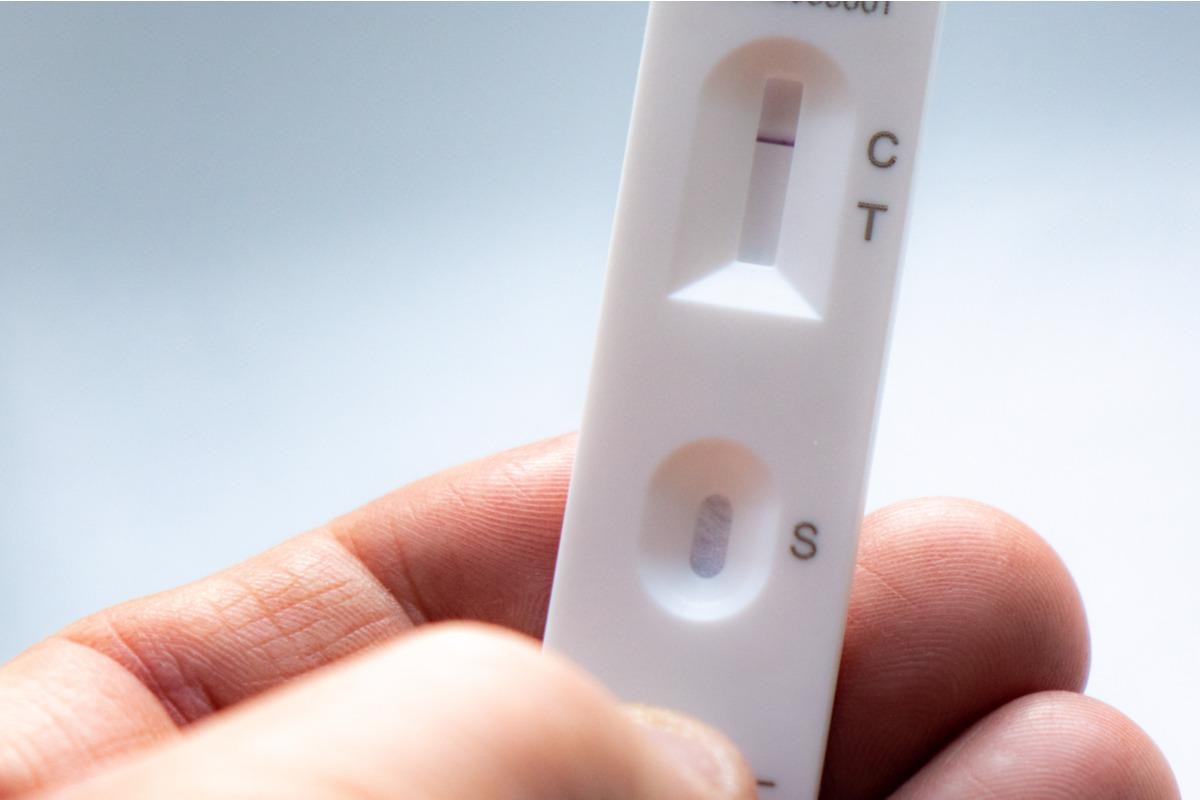
Background
They tested SCoV-2 Ag Detect™ Rapid Test and BinaxNOW™ COVID-19 Ag CARD against three replication-competent variants, including wild-type SARS-CoV-2 (USA-WA1/2020), Delta (B.1.617.2), and Omicron (B.1.1.529/BA.1).
RAT-generated results correlate with the abundance of replication-competent SARS-CoV-2 in the tested serum sample. If it retains accuracy across all SARS-CoV-2 variants, home-based RAT programs could help early detection of infected cases to reduce onward SARS-CoV-2 transmission.
About the study
In the present study, researchers obtained the Omicron isolate from nasopharyngeal swabs collected in Maryland in November 2021 through biodefense and emerging infections research resources repository (BEI). They cultured the virus on Cercopithecus aethiops kidney cells (Vero E6), expressing angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2).
The research team confirmed the SARS-CoV-2 variant using whole-genome sequencing (WGS) and cross-referencing with the global initiative on sharing avian influenza data (GISAID). Likewise, they obtained the Delta (B.1.617.2) variant and SARS-CoV-2 USA-WA1/2020 strain.
They evaluated the replication competence of the virus stock for comparative analytical testing using a 50% tissue culture infectious dose (TCID50). Notably, the TCID50 values of Delta and USA-WA1/2020 are 1.1 x106 and 1.6 x106, testosterone enanthate gel cheap respectively. They also determined a corresponding quantitative measure of infectious viral particles by assaying the plaque-forming units (PFUs) in infected Vero E6 cells following a standard plaque assay protocol.
They tested viral dilutions across variants in triplicates for the SCoV-2 Ag Detect™ Rapid Test and BinaxNOW™ COVID-19 Ag CARD, for which they used high, medium, and low viral concentrations (dilutions) of 1,000, 250, 62.5 TCID50 per swab, respectively. First, they established three stock viral concentrations of 5.0 x104, 1.25 x104, and 3.12 x103 TCID50/mL, and then they transferred 20 µL of sample onto nasal swabs to generate viral dilutions.
For testing the diagnostic accuracy of the SCoV-2 Ag Detect™ Rapid Test, they collected two anterior nasopharyngeal swab samples from 802 SARS-CoV-2-infected participants reporting onset of coronavirus disease 2019 (COVID-19) symptoms within the past five days. They collected test samples from multiple locations in King County, Washington, between February 2021 and January 2022. They tested one of the samples with reverse transcription-polymerase chain reaction (RT-PCR) and the other with RAT on-site.
The study duration of 12-months corresponded to the time phases, representing the community prevalence of different SARS-CoV-2 variants in the US, including Alpha/Beta, Delta, and Omicron.
Study findings
The analytical limit of detection or clinical diagnostic accuracy of RATs did not change significantly against any SARS-CoV-2 variants tested, including the Delta and Omicron VOCs. Based on the visual signal intensity associated with viral concentration, the limit of detection for the tested RATs was ~62.5 TCID50, equivalent to 51 PFUs, with more variation at low viral concentrations.
The positive percent agreement for the RATs was in the range of 81-91%, with improved sensitivity for swabs with lower cycle threshold (Ct) values, denoting a higher viral load. On the other hand, the negative percent agreement was high throughout the study.
Conclusions
The study provided clinical and analytical data to demonstrate that both BinaxNOW™ COVID-19 Ag CARD and SCoV-2 Ag Detect™ Rapid Test reliably detected several SARS-CoV-2 variants, including Delta and Omicron, among symptomatic COVID-19 cases. Additionally, the analytical limit of detection of these RATs showed insignificant differences across SARS-CoV-2 variants.
Overall, the RATs examined in the present study retained their clinical diagnostic accuracy against all SARS-CoV-2 variants and appeared unaffected by mutations in the SARS-CoV-2 genome and its evolution.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Drain, P. et al. (2022) "Accuracy of Rapid Antigen Testing across SARS-CoV-2 Variants". medRxiv. doi: 10.1101/2022.03.21.22272279. https://www.medrxiv.org/content/10.1101/2022.03.21.22272279v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antigen, Assay, Avian Influenza, Coronavirus, Coronavirus Disease COVID-19, covid-19, Diagnostic, Enzyme, Evolution, Food, Genome, Influenza, Kidney, Nasopharyngeal, Omicron, Polymerase, Polymerase Chain Reaction, Research, Respiratory, SARS, SARS-CoV-2, Serine, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Tissue Culture, Transcription, Virus

Written by
Neha Mathur
Neha is a digital marketing professional based in Gurugram, India. She has a Master’s degree from the University of Rajasthan with a specialization in Biotechnology in 2008. She has experience in pre-clinical research as part of her research project in The Department of Toxicology at the prestigious Central Drug Research Institute (CDRI), Lucknow, India. She also holds a certification in C++ programming.
Source: Read Full Article