Study explores favorable signatures for specific RNA motif recognition by SARS-CoV-2 nucleocapsid
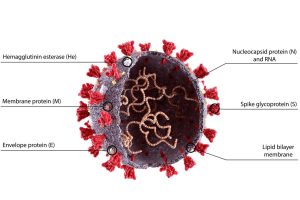
A recent article posted to the Research Square* preprint server and currently under consideration at a Nature Portfolio Journal, evaluated the N terminal domain (NTD) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid (N) protein. It presented the targeted characteristics for selective ribonucleic acid (RNA) identification by SARS-CoV-2 N in its 5'-genomic end (5'ge).
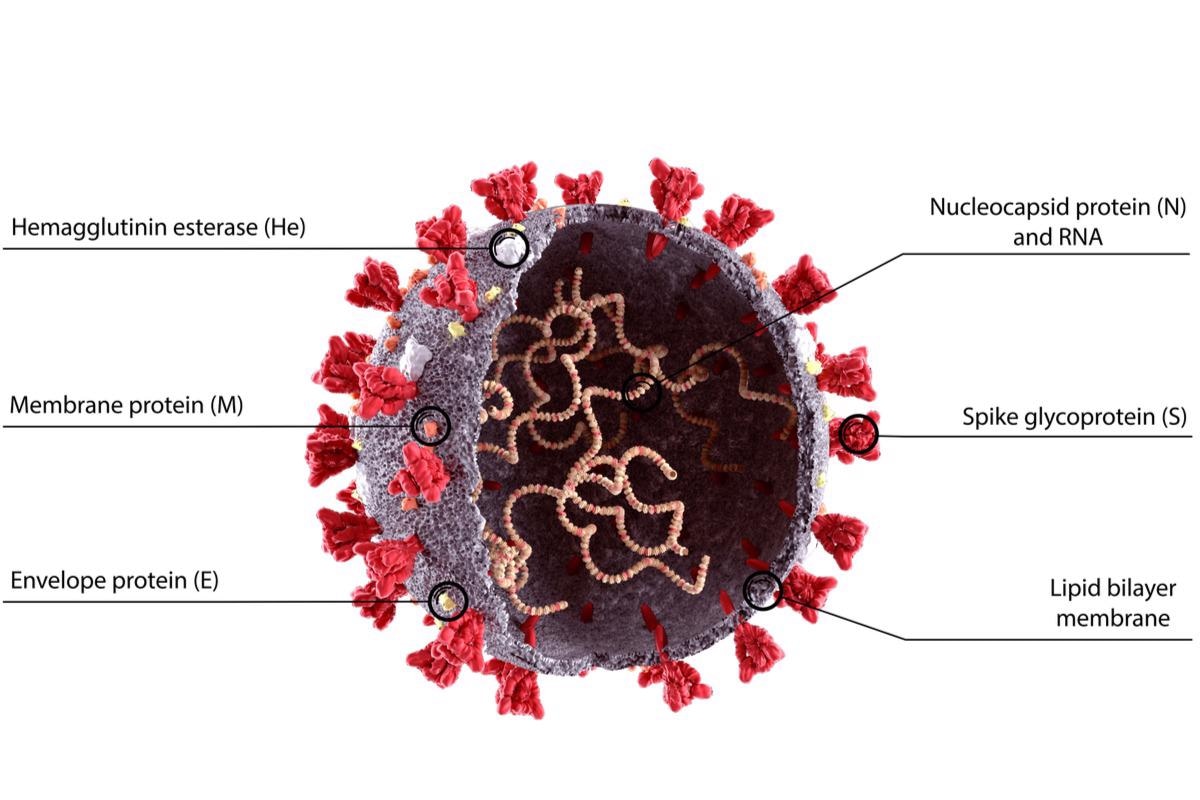
Background
The SARS-CoV-2 N protein performs a critical function during the viral life cycle. It is responsible for packing the vast genome into virus particles and is implicated in RNA transcription. N is also in charge of balancing the perplexing equilibrium between bulk RNA coating and meticulous RNA adherence to specific cis-regulatory regions. Although various investigations have revealed that N's disordered regions are involved in non-selective RNA identification, it is unclear how N regulates the obligatory recognition of RNA motifs.
About the study
In the present work, the researchers analyzed the contacts of RNA-binding NTD of the SARS-CoV-2 N with distinct cis-RNA elements clustered in the viral regulatory 5'ge using nuclear magnetic resonance (NMR) spectroscopy. Furthermore, the team demonstrated the stable and unique complexes between SARS-CoV-2 SL3/Ext and NTD using spectroscopies like circular dichroism (CD), small-angle X-ray scattering (SAXS), and size exclusion chromatography (SEC).
The NTD coding sequence of the SARS-CoV-2 N was based on NC_045512.2, National Center for Biotechnology Information (NCBI) reference genome entry, similar to GenBank entry MN90894. NTD of SARS-CoV-2 N was produced and purified by the authors for the current study. NMR quantifications were performed at the Frankfurt center for biomolecular magnetic resonance (BMRZ) employing Bruker spectrometers. All SAXS samples in the NTD buffer were quantified at room temperature. The PETRA III source was used to perform SAXS studies at the Deutsches Elektronen-Synchrotron (DESY) Hamburg's beamline P12.
RNA secondary structure models from the 5're RNAs SL1-SL6 were used for the study. Analytical SEC (aSEC) at 4°C was conducted. A Japan spectroscopic company (JASCO) J-810 spectropolarimeter with a Peltier temperature control module was utilized to detect the ultraviolet (UV) melting of several SCoV-2 5'ge RNAs employed in this investigation. The initial NTD RNA-binding preferences were analyzed using radioactive electrophoretic mobility shift assay (EMSA). The isothermal titration calorimetry (ITC) quantifications of NTD with SL4ext were performed on a VP-ITC200 instrument.
Results and discussions
The results demonstrated two components in the 5'-UTR of SARS-CoV-2, namely SL2+3 and Ext, that match with unique fingerprints in NMR observed binding to NTD and enhanced complex stability in aSEC. NTD's selectivity for both components was within the normal range for RBD-RNA complexes. In line with the prior reports, it was observed that the transcriptional regulatory sequence (TRS) was a selective target of NTD of the SARS-CoV-2's N. Furthermore, the current findings show that the Ext-RNA was bound by the NTD, confirming the results of previous works.
Both Ext and SL3 exist as transiently folded components, according to the CD and SAXS data. The researchers propose that the cis-RNAs' dynamic activity contributes to Ext and SL3 favored targetability by the NTD in the SARS-CoV-2 genome. The authors also state that the presence of stable structures like SL4 or SL2 influences the higher recognition of Ext and TRS. This emphasizes the importance of the genetic background in N's identification, which is yet poorly understood.
Ext was found to be a dynamic element in this study and was previously discovered as a key portion of a putative upstream ORF with SL4. This provides a whole new perspective on the regulatory hub SL4ext, emphasizing its significance in NTD favored recognition. Such a hub was favored by the NTD's general capacity to loosely connect with isolated SL4, where the domain unequivocally distinguishes between duplexed and bulged nucleotides despite its low selectivity. This demonstrates NTD's capacity to distinguish between neighboring genomic RNA motifs with great precision. Alternatively, when facing weak sites like SL4, the NTD may utilize preferred sites like Ext.
NTD did not have an active function in the direct unfolding of RNA. Instead, NTD consistently shifts Ext conformer ratios towards single-stranded RNA (ssRNA). Through this mechanism, N might conceal regulatory RNA elements from other RNA-binding proteins (RBPs)' access and prepares the RNA for packing or downstream activities. Thus, the findings highlight the importance of temperature for the fitness of SARS-CoV-2, which will regulate neuralgic RNAs equilibria to preferred interaction with N, using NTD contact with Ext.
Conclusions
The study findings uncovered the SARS-CoV-2 NTD RNA-binding selectivity in the natural genome setting via comprehensive solution-based biophysical information. The study shows that the domain reads the inherent characteristic of preferred RNA elements using a set of flexible sensory residues, enabling stable and selective compound formation within a broad pool of accessible motifs.
Overall, the current heteronuclear single quantum coherence spectroscopy (HSQC)-based evaluation of the SARS-CoV-2 NTD attachment to its 5’-UTR components enables a site-specific investigation of ribonucleoprotein (RNP) druggability employing more favorable RNA-NTD tandems. These findings shed light on the small changes in RNA motifs that are expected to account for NTD identification, including labile-fold and sequence requirements.
*Important notice
Preprints with Research Square publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Sophie Korn, Karthikeyan Dhamotharan, Andreas Schlundt, et al. (2022). The preference signature of the SARS-CoV-2 Nucleocapsid NTD for its 5’-genomic RNA elements. Research Square. doi: https://doi.org/10.21203/rs.3.rs-1445747/v1 https://www.researchsquare.com/article/rs-1445747/v1
Posted in: Medical Research News | Medical Condition News | Disease/Infection News
Tags: Assay, Biotechnology, Chromatography, Compound, Coronavirus, Coronavirus Disease COVID-19, Genetic, Genome, Genomic, Nucleotides, Protein, Regulatory RNA, Research, Respiratory, Ribonucleic Acid, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spectroscopy, Syndrome, temperature control, Titration, Transcription, Virus, X-Ray

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article