New model brings researchers closer to growing natural bypasses for blocked hearts
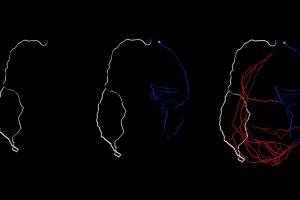
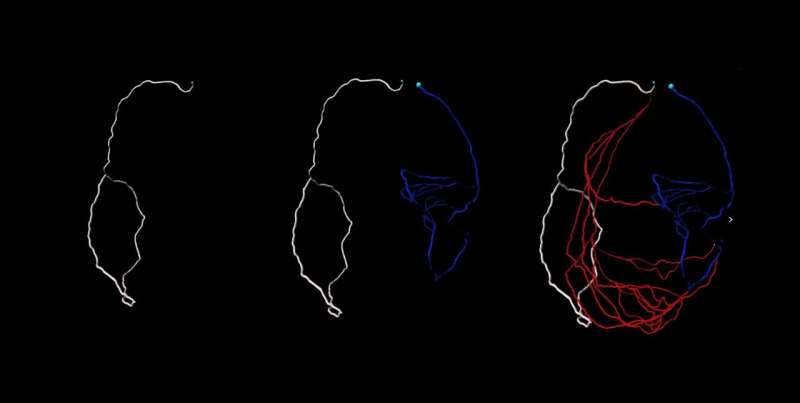
Some people and animals grow arteries that allow blood to bypass blockages in the heart. But to engineer these bypasses to serve as lifelines, scientists first need to understand how they work. Researchers have now modeled blood flow through small vessels surrounding the heart.
Guinea pigs are heart-attack proof. With large coronary arteries that bypass the heart, they can survive the sorts of blockages that kill millions of people every year. Now researchers are working to spur the growth of such arteries to treat people with heart disease.
Blood vessels typically branch like trees, but these bypasses, called collateral arteries, are special, says developmental biologist Kristy Red-Horse, a Howard Hughes Medical Institute Investigator at Stanford University. “They connect like ladder rungs to larger arteries,” allowing them to carry oxygenated blood to the tissue downstream of a blockage.
About 20 to 30 percent of people have some flow through collateral arteries. “We can use the heart’s natural mechanisms and push it toward making its own natural bypasses,” Red-Horse says. That could lead to therapies to replace invasive interventions such as coronary artery bypass surgery. But to engineer these blood vessels to be lifelines, scientists first need to understand their blood flow. Now, Red-Horse and colleagues have imaged dozens of mouse hearts and created a detailed model based on the physics of blood flow. Their model has revealed an arterial arrangement that mimics that of newborns, not adults, enables collaterals to more successfully circumvents blockages, the researchers report August 12, 2022, in Nature Cardiovascular Research.
Collateral arteries are akin to an exit on a freeway that provides a way for cars to move around an accident. But it’s not enough that alternate routes exist—they must be big enough to allow cars to get where they need to go quickly. When blood stalls in arteries like a freeway jam, the consequences can be fatal.
Red-Horse and her team imaged whole hearts from newborn and adult mice to view how collateral arteries are laid out at different stages of life. Days to weeks after these mice experienced heart attacks, the animals grew collateral arteries. Then the team imaged their organs, adapting the iDISCO method created by HHMI Investigator alum Marc Tessier-Lavigne, who is now President of Stanford University.
These techniques have unlocked the ability to image the entire heart and capture every single coronary artery—”a roadblock for a very long time,” Red-Horse says. Red-Horse, whose lab specializes in imaging coronary blood vessels, teamed up with Stanford University bioengineer and Douglass M. and Nola Leishmann Professor of Cardiovascular Disease, Alison Marsden, to develop a computer model for blood flow based on their microscopy images.
“This is the first time that blood flow has been modeled in the small coronary arteries,” Red-Horse says. “We could never have done it if it weren’t for the interdisciplinary approach and the trainees that took on the challenging task of merging expertise from two very disparate fields.” The work was co-led by Ph.D. students, Suhaas Anbahakan and Pam Rios Coronado, and required key Stanford collaborators, Daniel Bernstein, Koen Nieman, and Anca Pașca.
That model provided new insights into how blood flow differs between newborn and adult hearts. Newborns’ collateral arteries outmatched the adults’ arteries at carrying blood after a heart attack. This ability to swiftly bring fresh blood may contribute to newborn hearts’ capacity to regenerate. Newborns can completely regenerate their hearts after a heart attack. “Adult hearts cannot, and that’s why heart disease is such a large problem in our society.”
At birth, the coronary arteries’ main highways are laid down. Then across life, the heart grows to 10 times its neonatal size. But, the team saw, those highways don’t widen—later in life, the coronary artery tree just added more side streets.
The team compared their results from mice with humans, imaging five fetal hearts and scoping out the arteries of adults whose hearts had been imaged by X-rays. Some people, particularly those with slowly-developing heart disease, develop functional collateral arteries, which shunt flow around a blockage. The adult heart disease patients each had at least two collateral arteries. Surprisingly, each fetal heart had more than 40 collateral arteries. “During embryonic development, we have the capacity to make a huge number of these arteries,” Red-Horse says.
“Red-Horse’s work provides a cellular blueprint for understanding collateral artery formation and the underlying molecules and mechanisms,” says Eric Olson, a molecular biologist at University of Texas Southwestern Medical Center who was not part of the work. “Leveraging this knowledge can provide new strategies to reawaken the neonatal regenerative response in adulthood” after a heart attack, he says.
Red-Horse and her colleagues have already started searching for ways to kickstart the growth of collateral arteries. In previous work, her team injected mice with chemokines, protein signals, that instruct the heart to make new bypasses. And someday it may be possible to deliver these proteins non-invasively and before a heart attack even occurs.
Source: Read Full Article