New ovarian cancer screening technique shows reduced false negatives compared to CA125 method
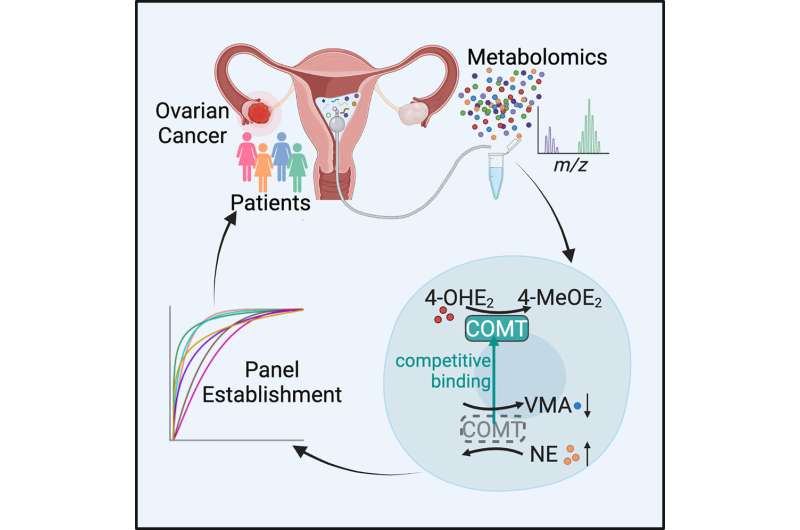
Researchers at the Department of Obstetrics and Gynecology, Peking University Third Hospital in Beijing, China, have developed a new ovarian cancer screening technique. In a paper, “Profiling the metabolome of uterine fluid for early detection of ovarian cancer,” published in Cell Reports Medicine, the research team details the markers and methods used to develop the test.
The researchers collected uterine fluid from 219 patients with various stages of ovarian cancer or benign gynecological diseases. No healthy patients were tested. The analysis of 1,213 metabolites was narrowed to just seven with significant associations with ovarian cancer.
In testing diagnostic ability, the study finds an overall 88% accuracy with their seven-marker method compared to 79% with the existing CA125 blood marker test.
The researchers suggest their study identifies specific metabolites as downstream indicators of genes and proteins involved in tumor initiation and “…not only reveals metabolic features in uterine fluid of gynecological patients but also establishes a noninvasive approach for the early diagnosis of ovarian cancer.”
While the test illustrates a lower level of false negatives compared to existing blood tests, a robust control group of individuals without cancer or benign gynecological diseases is missing from the study.
Without the healthy control, the rate of false positives with the test is unclear. A control group of at least five healthy individuals for every pathology case in the study would usually be expected and is absent from the current study. Future follow-up research may clarify this issue.
The challenge for any pre-diagnosis disorder screening method is identifying a disease and avoiding false positives. While a test only taken by people with diagnostic signs of a disease can be a significant confirmation, screening to catch disease markers before a diagnosis means that the screening method is applied to mostly healthy populations.
Ovarian cancer will only affect around 1.3% of women in a population (at some point in their lifetime), so in a population of 1,000 people screened, the hope would be to find the 13 (or less because the 1.3% risk is over a lifetime) at risk for early signs of the disease.
Suppose the test has a false positive rate of 12%. In that case, within the same population of 1,000, 120 additional women without risk would also test positive, making the test less than 10% accurate and potentially causing alarm and unneeded invasive diagnostic procedures.
If given to the general public, a negative test result would be around 98% reliable, even with false positive and false negative rates of 12%. That rate drops considerably when the test is targeted only at patients with diagnostic signals, as around 12% will get false negative results.
It is unlikely that a stand-alone ovarian cancer diagnostic screening tool for a general population has been found, but further research may prove otherwise.
More information:
Pan Wang et al, Profiling the metabolome of uterine fluid for early detection of ovarian cancer, Cell Reports Medicine (2023). DOI: 10.1016/j.xcrm.2023.101061
Journal information:
Cell Reports Medicine
Source: Read Full Article