prk vs lasix
Hospital nurseries routinely place soft bands around the tiny wrists of newborns that hold important identifying information such as name, sex, mother, and birth date. Researchers at Rockefeller University are taking the same approach with newborn brain cells—but these neonates will keep their ID tags for life, so that scientists can track how they grow and mature, as a means for better understanding the brain’s aging process.
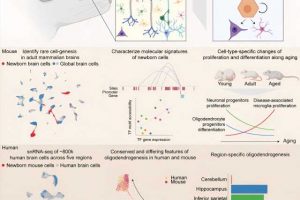
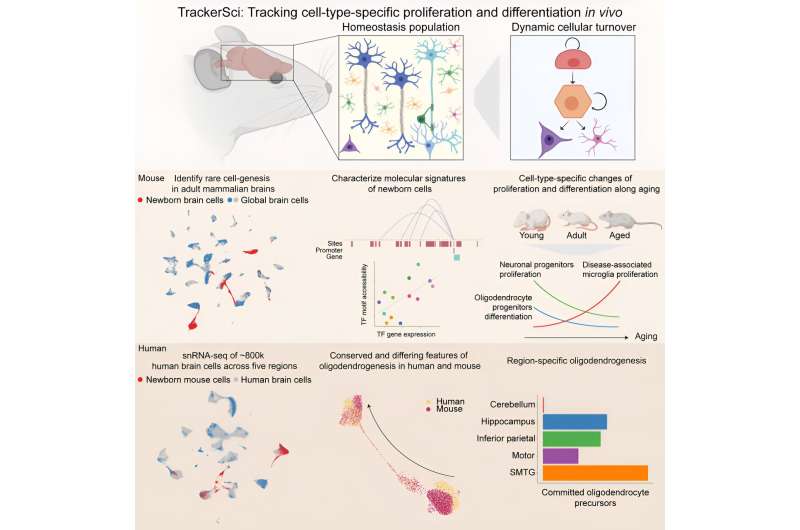
As described in a new paper in Cell, vicodin 5mg 325 the new method developed by Rockefeller geneticist Junyue Cao and his colleagues is called TrackerSci (pronounced “sky”). This low-cost, high-throughput approach has already revealed that while newborn cells continue to be produced through life, the kinds of cells being produced greatly vary in different ages.
This groundbreaking work, led by co-first authors Ziyu Lu and Melissa Zhang from Cao’s lab, promises to influence not only the study of the brain but also broader aspects of aging and disease across the human body.
“The cell is the basic functional unit of our body, so changes to the cell essentially underlie virtually every disease and the aging process,” says Cao, head of the Laboratory of Single-Cell Genomics and Population Dynamics. “If we can systematically characterize the different cells and their dynamics using this novel technique, we may get a panoramic view of the mechanisms of many diseases and the enigma of aging.”
Rare and powerful
New cells are continuously produced in the adult mammalian brain, a critical process associated with memory, learning, and stress. They develop from progenitor cells—descendants of adult stem cells that differentiate into specialized cell types.
How this process unfolds, however, has been largely unknown, both because of technological limitations and cell rarity. Finding progenitor cells in the brain is a needle-in-haystack endeavor; in mammals, they account for a mere .5 percent of all brain cells. That number drops to .1 percent in later stages of life—a downward shift due to cellular instability, a core characteristic of disease and aging.
Cao studies how tissues and organs maintain stable populations of cells—a hallmark of health—so he and his team wanted to investigate how different cellular populations develop, and whether these varied neuronal cells decline in the same way or forge different paths. Tracking their cellular lifespans from birth to maturity would reveal not just differences, but also when they appeared.
His lab specializes optimizing methods for single-cell sequencing, an increasingly popular approach to analysis that homes in on the genetic expression and molecular dynamics of individual cells. Cao’s group uses combinatorial indexing, a sophisticated yet cost-effective technique that allows for the simultaneous analysis of millions of cells.
This method uniquely tags cellular molecules with distinct barcodes that correlate to each cell’s unique molecular assembly. With TrackerSci, Cao and his colleagues have fine-tuned this technique even further. This enhancement enables the meticulous labeling and tracking of the dynamics of rare progenitor cells in mammalian organs.
“It’s like an ID card and GPS tracker combined,” Cao says.
Younger vs. older
For the current study, the researchers analyzed more than 10,000 newborn progenitor cells from across entire mouse brains spanning three ages (young, mature, and elderly) with a synthetic molecule known as 5-ethynyl-2-deoxyuridine (EdU). As these newborn cells differentiated, proliferated, and dispersed, EdU continued to label their DNA, functioning like a GPS tracker.
This innovative technique allowed the researchers to analyze tens of thousands of gene expressions and the chromatin landscapes of these newborn cells as they grew into families of cell types with different molecular functions.
“We were able to quantify cellular proliferation and differentiation rates of many cell types across the entire brain in a single experiment, which wasn’t possible using conventional approaches,” Cao says. “Those only capture static information—the current molecular state of a cell at a single moment. But TrackerSci captures dynamic information over time. It’s like other methods take snapshots, and we shoot a film.”
Some clear—and surprising—characters emerged from these movies. Most strikingly, there were radical shifts in the type of cells generated, depending on the age of the mouse.
For example, the number of progenitors that become neurons, the essential communicative cells of the brain, are higher in young brains. The same is the case for a range of glial cells, which create a stable environment for neurons by ensheathing them, providing nutrients, and defending against pathogens—all important for a young, still-developing organ.
The opposite is true in the elderly brain. Progenitor cells rarely become either neurons or glial cells; in fact, virtually every type of brain cell plummets. Most lost are dentate gyrus neuroblasts, which are essential for creating neurons in the hippocampus, a region linked to memory and diseases like Alzheimer’s. In comparison to the adult brain, the number of these cells drops by 16-fold in the elderly brain.
Instead, immune cells and microglia, a kind of macrophage, proliferate in the aging brain. But rather than protect the brain, they convert into an inflammatory cellular state specific to aging—and these cells are produced at a higher rate. In short, the aging brain creates more of the cells that create more problems for the aging brain.
The sci’s the limit
Cao says TrackerSci could be used to track the regenerative capacity of many organs.
“We’re not a brain lab,” he notes. “We also tested the protocol for profiling progenitor cells in the lung, colon, pancreas, and many different organs.”
Other organs have far higher proportions of progenitor cells than brains do; newborn progenitors account for more than 20 percent of the cells in the colon, for instance. A few years ago, Cao demonstrated the potential for analyzing cell population dynamics in human fetal development by creating a cellular atlas using a similar combinatorial indexing method.
TrackerSci is one of several single-sequencing techniques to recently emerge from Cao’s lab. Another, called PerturbSci-Kinetics, developed by graduate student Zihan Xu, decodes the genome-wide regulatory network that underlies RNA temporal dynamics by coupling scalable single-cell genomics with high-throughput genetic perturbations, or manipulations that can influence gene function. The method was recently described in a paper in Nature Biotechnology.
More information:
Ziyu Lu et al, Tracking cell-type-specific temporal dynamics in human and mouse brains, Cell (2023). DOI: 10.1016/j.cell.2023.08.042
Zihan Xu et al, Dissecting key regulators of transcriptome kinetics through scalable single-cell RNA profiling of pooled CRISPR screens, Nature Biotechnology (2023). DOI: 10.1038/s41587-023-01948-9
Journal information:
Nature Biotechnology
,
Cell
Source: Read Full Article