prilosec long term usage
Until the COVID-19 pandemic exploded, few people outside of research labs and intensive care units had heard of a cytokine storm. But once this dangerous form of infection-triggered runaway inflammation started claiming lives by the thousands, cytotec venta en farmacias argentina a legion of scientists jumped into the hunt for ways to calm these storms.
Now, a study led by immunobiology experts at Cincinnati Children’s, offers important new details on how two elements of our body’s immune system clash with each other to prompt a chain of reactions that can release deadly floods of cell-killing, organ-damaging cytokines. Details were published Oct. 3, 2023, in the journal Cell Reports.
“These findings have implications for both autoimmunity as well as cancer,” says corresponding author Chandrashekhar Pasare, DVM, Ph.D., director of the Division of Immunobiology and co-director of the Center for Inflammation and Tolerance at Cincinnati Children’s. “We have discovered an independent cell signaling pathway that allows a type of immune cell called an effector memory T cell (Tem) to become a critical driver of innate cytokine storms.”
With a pathway defined, next steps include confirming whether medications that target key points along the pathway can disrupt the inflammation cycle before it becomes uncontrollable, Pasare says. That will require further study.
Damaged dendritic cells
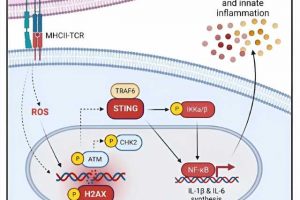
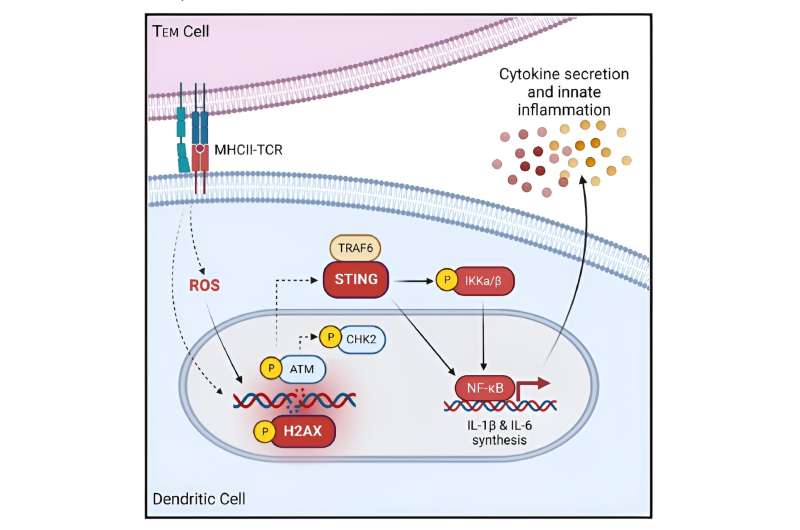
The chain reaction appears to start when Tem cells interact with dendritic cells, which serve as the immune system’s primary detector of viral and bacterial invasions. These starburst shaped cells carry a large collection of receptors to detect various types of invaders. Once they encounter a harmful visitor, dendritic cells latch on and sound an alarm that instructs the rest of the immune system’s machinery to begin producing T cells that are custom designed to eliminate that type of invader.
When the immune system wins the battle, most of the custom T cells stand down. But a few guards linger in the blood and other body tissues to be ready to “effect” a rapid response should the same type on infection occur again. Hence the name effector memory T cells.
However, the research team discovered that ongoing encounters with Tem cells, such as those occurring when people have autoimmunity or live in a state of chronic inflammation, actually cause DNA strands within dendritic cells to break. This, in turn, prompts a DNA repair pathway that rapidly generates large numbers of inflammatory cytokines, including IL-1b, IL-6 and IL-12.
This flood, or storm, of cytokines causes the tissue damage that occurs in autoinflammatory diseases including type 1 diabetes, multiple sclerosis, rheumatoid arthritis, and inflammatory bowel diseases like Crohn’s disease. For some people with these conditions, ongoing inflammation also increases their risk of developing cancer.
Removing the STING
Pasare and colleagues worked for the last 10 years to examine the genetic activity and cell-to-cell communications occurring behind this process. Collaborators from Cincinnati Children’s included first author Hannah Meibers, Ph.D., and contributions from Kathrynne Warrick, MD-Ph.D. student, Andrew VonHandorf, Ph.D., Charles Vallez, MD-Ph.D. student, Kiana Kawarizadeh, BS, Irene Saha, Ph.D., Omer Donmez, MS, Viral Jain, MD, (now with the University of Alabama at Birmingham), Leah Kottyan, Ph.D., and Matthew Weirauch, Ph.D.
The team found a surprising clue when examining the transcriptional profile of dendritic cells following their interaction with Tem cells. Specifically, they detected upregulation of expression of Tmem173, which encodes for stimulator of interferon genes (STING). The STING pathway has been described in previous research as being important to detect viral infections. But when Tem cells harm dendritic cells, the STING pathway does not follow the same route that it typically does when directly responding to viral infections.
In this situation, STING teams up with the gene TRAF6 and the transcription factor NFkB to form an “axis” of activity that drives runaway production of innate inflammatory cytokines.
The researchers further reasoned that if they could prevent STING and TRAF6 from working together, they could cut off the inflammation chain reaction at an early stage. In mice gene-edited to lack the STING pathway, that’s exactly what they found. When treated with a drug known to induce an intense T cell-mediated inflammatory response, these mice did not produce a flood of innate cytokines.
Challenges ahead
The mouse study involved a whole-body elimination of STING. Attempting the same in humans would not be advisable because STING is used by a number of cell types outside the immune system in necessary ways, Pasare says.
“Our goal will be to develop a highly focused method or methods for blocking STING within targeted immune cells, without disrupting its other important functions,” Pasare says. “If we can achieve that, we may have a powerful new tool for controlling hyper-inflammation.”
More information:
Hannah Meibers et al, Effector Memory T cells induce innate inflammation by triggering DNA damage and a non-canonical STING pathway in dendritic cells, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113180. www.cell.com/cell-reports/full … 2211-1247(23)01192-0
Journal information:
Cell Reports
Source: Read Full Article