Researchers solve mystery behind antibiotic-resistant C. difficile infection
Researchers at the Texas A&M University Health Science Center (Texas A&M Health) have uncovered why C. diff (also known as Clostridioides difficile or C. difficile) infection has developed resistance to the antibiotic metronidazole.
C. diff is a bacterium that infects the colon and causes diarrhea and colitis, or inflammation of the colon. It causes almost half a million infections in the United States each year and is the leading cause of hospital-acquired illness in the United States. The Centers for Disease Control and Prevention estimates that one in 11 adults over age 65 diagnosed with hospital-associated C. diff die within one month.
C. diff has challenged physicians for decades. One reason why it is so common in health care settings is because it appears after a patient has been treated with broad-spectrum antibiotics for other infections. These antibiotics disrupt the gastrointestinal tract and cause dysbiosis, or an imbalance of the gut microbiome, which then allows C. diff to come in, colonize and cause diarrhea.
Fluoroquinolones, which include ciprofloxacin (Cipro), are a class of broad-spectrum antibiotics to which certain strains of C. diff are highly resistant. These fluoroquinolone-resistant strains are responsible for a global outbreak of C. diff throughout the early 2000s.
During the 1980s and 1990s, the antibiotic metronidazole was successfully used to treat C. diff infections. Back then, metronidazole had a greater than 90% success rate, but that has declined rapidly over the last two decades to almost as low as 60%. This decline coincided with the 2000s outbreaks of fluoroquinolone-resistant C. diff across the western world, including North America, the United Kingdom, Europe and Latin America.
During this time, metronidazole and vancomycin were the main two antibiotics used to treat C. diff infection, until fidaxomicin was approved by the Food and Drug Administration in 2011.
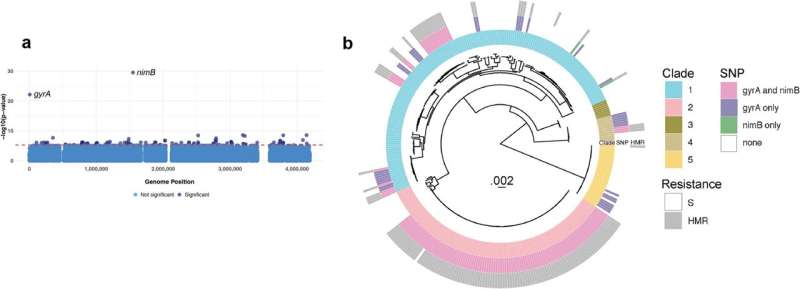
Until now, it’s been unclear exactly how metronidazole has become less effective against fluoroquinolone-resistant C. diff. But a research team led by Dr. Julian Hurdle from the Texas A&M Health Institute of Biosciences and Technology has finally unmasked the genetic reason.
In a study published in Nature Communications, the team describes a mutation these strains of C. diff have acquired in a gene known as 5-nitroimidazole reductase, or nimB for short. The nimB enzyme essentially chews up metronidazole and inactivates it. That mutation occurs in the gene’s promoter, which controls expression, causing an increase in production of nimB.
“The bacteria that had spread around the world were resistant to fluoroquinolone—that’s a selection pressure, or forced evolution, in the hospital—but subsequently had resistance to metronidazole due to this upregulation of a gene that chews up metronidazole,” Hurdle said.
Now that the genetic mechanism for metronidazole resistance in C. diff has been identified, Hurdle says a molecular diagnostic test can be developed to identify when a patient is infected with that strain of C. diff.
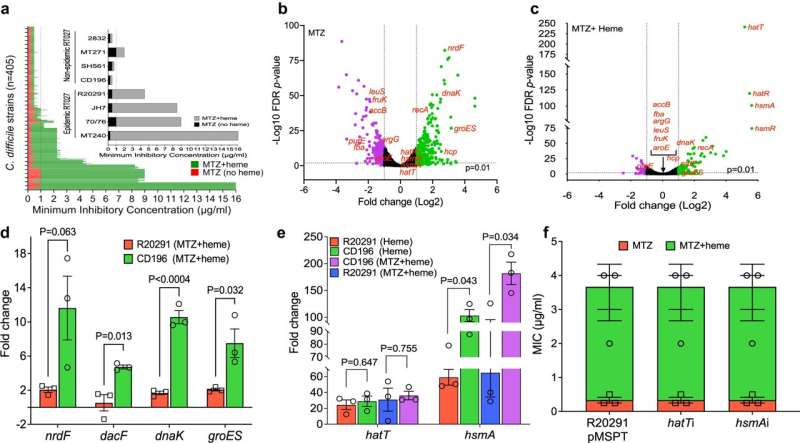
For other types of infections, such as staphylococcus—which can cause methicillin-resistant Staphylococcus aureus (MRSA) infections—molecular diagnostics can be used to identify the genetic mechanism of resistance in the genome. That test tells clinicians whether the patient has a tetracycline-resistant staph, for example, so they can avoid introducing that class of drugs and instead start a course of antibiotic that has a better chance of effectively treating it.
Up until now, a molecular diagnostic could not be used for metronidazole-resistant C. diff because scientists didn’t know what genetic mechanism the test needed to look for.
“One of the things in the paper we wanted to point out is that if we knew long ago what the mechanism was, we could have easily come up with a molecular diagnostic for metronidazole,” Hurdle said. “When you’ve gone through a course of antibiotics and the patients go on to have a recurrence of C. difficile infection, then you’re chasing after clinical success, and it becomes harder and harder to treat. Having a molecular diagnostic will save patient mortality. It will save lives.”
Because of its declining efficacy, metronidazole is no longer recommended as a first-line treatment for C. diff infection in the updated clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). However, the inexpensive drug is still sometimes used, especially outside of the United States.
“Those guidelines are based on clinical patient data, but no one has yet been able to attach, specifically, that metronidazole is less effective because of resistance, and that is what we show with this study,” Hurdle said.
The next drug Hurdle’s team is evaluating is vancomycin. “I think we may have already lost metronidazole as a treatment for C. diff and could lose vancomycin as well, since resistance to this drug has also occurred,” he said. If clinicians can intervene earlier with molecular diagnostics to identify patients with vancomycin-resistant C. diff infection, Hurdle is hopeful that patients can be better treated and another epidemic-level outbreak of the bacterium can be avoided.
More information:
Abiola O. Olaitan et al, Decoding a cryptic mechanism of metronidazole resistance among globally disseminated fluoroquinolone-resistant Clostridioides difficile, Nature Communications (2023). DOI: 10.1038/s41467-023-39429-x
Journal information:
Nature Communications
Source: Read Full Article