Lowering a form of brain cholesterol reduces Alzheimers-like damage in mice
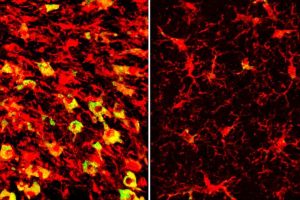
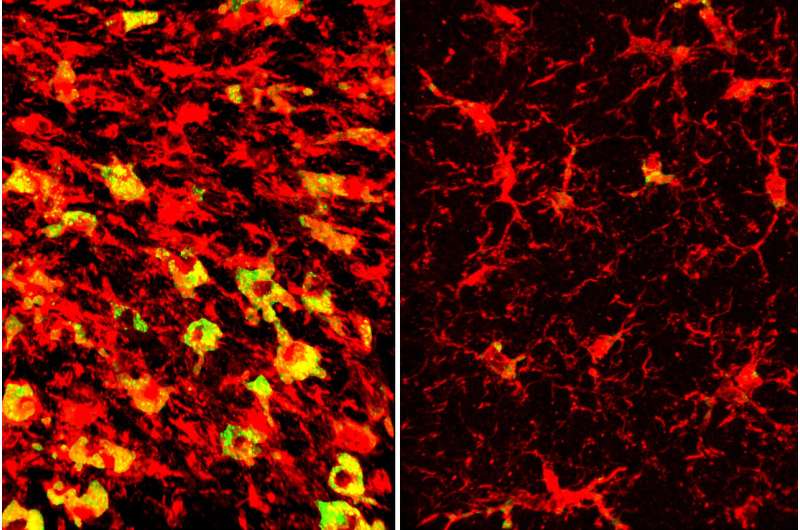
In Alzheimer’s disease and related dementias, cognitive decline is driven by the over-accumulation of a normal brain protein known as tau. Wherever tau builds up, nearby brain tissue starts to degenerate and die.
Now, researchers at Washington University School of Medicine in St. Louis have found—in mice—that Alzheimer’s-like tau deposits in the brain lead to the accumulation of a form of cholesterol known as cholesteryl esters, and that lowering cholesteryl ester levels helps prevent brain damage and behavioral changes.
“This has important therapeutic implications,” said senior author David M. Holtzman, MD, the Barbara Burton and Reuben M. Morriss III Distinguished Professor of Neurology.
“The compound we used in this study has side effects that make it unsuitable for use in people. But if you could develop a therapy that reduces cholesteryl esters inside brain cells without unacceptable side effects, it would be a promising candidate to test in neurodegenerative diseases.”
The findings were published Nov. 22 in the journal Neuron.
The link between cholesterol and dementia is not as far-fetched as it might seem. The biggest genetic risk factor for Alzheimer’s is APOE, a gene involved in activating the brain’s immune cells. When such cells are activated in the wrong way or at the wrong time, they can damage brain tissue. But APOE also has another important job in the body: It carries cholesterol and other lipids around in the blood. In this capacity, it plays a role in atherosclerosis.
To investigate the connections between APOE, lipids and brain damage, Holtzman and first author Alexandra Litvinchuk, Ph.D., a postdoctoral researcher, studied mice with a high-risk tau gene that predisposes them to accumulate tau in their brains. Such mice start developing signs of neurodegeneration around 6 months of age.
By 9½ months, their brains are severely damaged, and they no longer are able to complete ordinary tasks of mouse life such as properly building a nest. The mice also carried a second genetic modification: Their own APOE genes had been removed and either replaced with a variant of the human APOE gene—APOE3, which confers an average risk of Alzheimer’s; or APOE4, which doubles or triples the risk of Alzheimer’s—or not replaced at all.
Investigation revealed that APOE4 is linked to distorted lipid metabolism in the brain. In 9½-month-old tau mice carrying APOE4, the same brain areas that became atrophied and damaged also amassed excess lipids, and in a strange pattern. Levels of more than 180 kinds of lipids were altered.
Among the most striking differences was that immune cells known as microglia in those areas were filled to the brim with cholesteryl esters. APOE3 did not have the same effect. The measurement of the brain lipids was done in collaboration with scientists at the company Denali Therapeutics led by Gilbert Di Paolo, Ph.D.
“Microglia filled up with lipids become hyperinflammatory and start secreting things that are not good for the brain,” Holtzman said.
Therefore, clearing out lipids potentially could reduce brain inflammation and neurodegeneration, he said. To find out, Litvinchuk and Holtzman used an LXR agonist, a member of an experimental class of drugs that lowers lipid levels in cells. The researchers fed the drug, called GW3965, to tau mice carrying APOE4, starting at 6 months of age.
The mice were assessed at 9½ months, by which point their brains normally would have sustained considerable damage. Mice that had received the drug retained significantly more brain volume than those that had received a placebo. They also had lower levels of tau, fewer inflammatory cells and less inflammation, less loss of synapses in their brains, and were better at building nests.
Further investigation revealed that the LXR agonist works by upregulating a gene called Abca1 that helps move cholesterol and other lipids out of cells. Using genetic methods to increase Abca1 levels had the same effect as drug treatment: less lipid accumulation, lower levels of tau, less inflammation and reduced neurodegeneration.
“What’s exciting is that we see all these effects in an animal model that shares a lot of features with human neurodegenerative diseases,” Holtzman said. “It shows that this kind of approach could have a lot of promise.”
One major obstacle stands in the way of translating this approach to people, Holtzman added. LXR agonists also affect lipid metabolism in the liver, and so they tend to cause fatty liver disease. Chemists are hard at work trying to design LXR agonists without that side effect. If they succeed, the resulting drugs may have benefits for heart disease as well as brain disease.
“There’s a lot of similarity between the mechanism that’s driving immune cells to damage the brain in Alzheimer’s disease and the one that’s driving the same kinds of immune cells to cause vascular damage in atherosclerosis,” Holtzman said.
“In both cases, lipids accumulate in immune cells, causing them to become hyperinflammatory and damage nearby tissues. Getting rid of that lipid accumulation may have double benefits for human health.”
More information:
Alexandra Litvinchuk et al, Amelioration of Tau and ApoE4-linked glial lipid accumulation and neurodegeneration with an LXR agonist, Neuron (2023). DOI: 10.1016/j.neuron.2023.10.023. www.cell.com/neuron/fulltext/S0896-6273(23)00804-8
Journal information:
Neuron
Source: Read Full Article