Tapping the brain to boost stroke rehabilitation

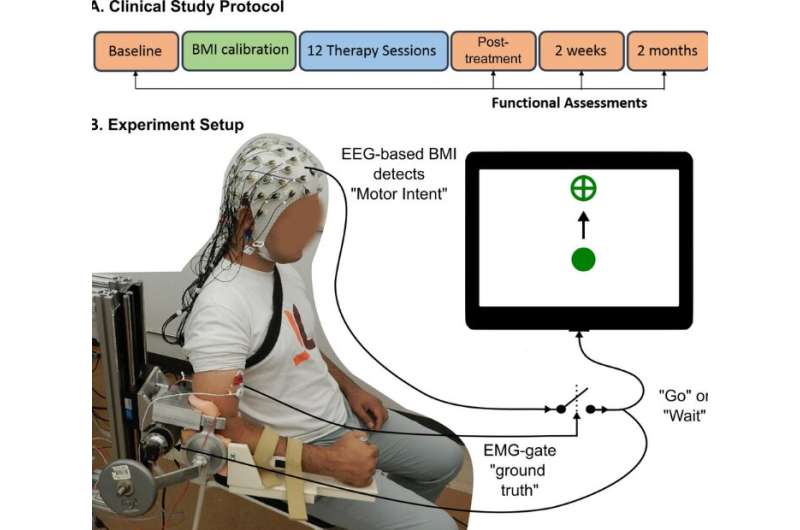
Stroke survivors who had ceased to benefit from conventional rehabilitation gained clinically significant arm movement and control by using an external robotic device powered by the patients’ own brains.
The results of the clinical trial were described in the journal NeuroImage: Clinical.
Jose Luis Contreras-Vidal, director of the Non-Invasive Brain Machine Interface Systems Laboratory at the University of Houston, said testing showed most patients retained the benefits for at least two months after the therapy sessions ended, suggesting the potential for long-lasting gains. He is also Hugh Roy and Lillie Cranz Cullen Distinguished Professor of electrical and computer engineering.
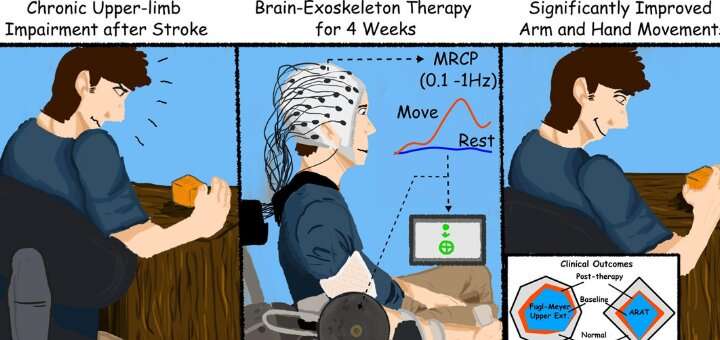
The trial involved training stroke survivors with limited movement in one arm to use a brain-machine interface (BMI), a computer program that captures brain activity to determine the subject’s intentions and then triggers an exoskeleton, or robotic device affixed to the affected arm, to move in response to those intentions. The device wouldn’t move if intention wasn’t detected, ensuring subjects remained engaged in the exercise.
Using robotics in rehabilitation isn’t new, said Contreras-Vidal, co-principal investigator of the trial and a pioneer in noninvasive BMI systems. But robot-assisted exercise doesn’t generally engage the user, which is critical for taking advantage of the brain’s plasticity to allow patients to relearn movement.
“This project ensures the brain is engaged,” he said. “We know that if the arm is moving, it’s because they are commanding it to move. That’s a very powerful concept.”
By testing the subjects over a period of time before the trial began, researchers were able to ensure that any changes or improvements were due to the intervention. In addition to better arm movement, the researchers reported that the subjects also showed improvements in using their hands.
“This is a novel way to measure what is going on in the brain in response to therapeutic intervention,” said Dr. Gerard Francisco, professor and chair of physical medicine and rehabilitation at McGovern Medical School at The University of Texas Health Science Center at Houston and co-principal investigator. “This study suggested that certain types of intervention, in this case using the upper robot, can trigger certain parts of brain to develop the intention to move. In the future, this means we can augment existing therapy programs by paying more attention to the importance of engaging certain parts of the brain that can magnify the response to therapy.”
The trial was conducted at TIRR Memorial Hermann, where Francisco serves as chief medical officer and director of the NeuroRecovery Research Center. The project was a collaboration between UH, UTHealth, TIRR Memorial Hermann, Houston Methodist Research Institute and Rice University.
In addition to Francisco and Contreras-Vidal, who is also director of the BRAIN Center, a NSF Industry/University Collaborative Research Institute, researchers involved with the project include Nikunj A. Bhagat and Zachary Hernandez with UH; Nuray Yozbatiran and Rupa Paranjape with UTHealth; Dr. Zafer Keser, formerly with UTHealth; Jennifer L. Sullivan, Colin Losey and co-principal investigator Marcia K. O’Malley with Rice; and Dr. Robert Grossman with Houston Methodist Research Institute. O’Malley is also Director of Rehabilitation Engineering at TIRR Memorial Hermann.
“Those of us who have studied the brain for so many years have anticipated that its powers, combined with robotics and the brain-machine interface, could offer unimaginable benefits to stroke survivors and other patients with brain injuries,” said Grossman, professor of neurosurgery at Houston Methodist. “This study is just the beginning of what will be possible to treat stroke, spinal cord injuries and other traumatic brain injuries in the future.”
The trial spanned a period of several years, partly because it took time to find subjects who met the criteria and were both interested in participating and able to make the required time commitment. Ultimately, 10 subjects between the ages of 41 and 71 were enrolled.
The therapy took place three times a week for four weeks. The final follow-up testing was conducted two months after therapy ended, and Contreras-Vidal said it’s unclear if the benefits will persist long-term.
That leads to an ongoing project—Contreras-Vidal has a National Science Foundation grant to design a low-cost system that would allow people to continue the treatments at home.
Source: Read Full Article