Single Sputnik V vaccine dose elicits robust immune response in previously infected individuals

As the coronavirus disease 2019 (COVID-19) pandemic became a raging outbreak in multiple countries, the effects of non-pharmaceutical interventions (NPIs) on social and economic life threatened to become intolerable and unsustainable. This led to intensive research on vaccines to build population immunity against the pathogen responsible, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The successful development of multiple vaccines and their emergency use authorization by American and European health regulatory agencies have resulted in their rollout in many countries the world over, in a period of approximately one year from conception.
A new preprint research paper posted to the medRxiv* server describes successful immunization of the vaccinated population with a single dose of the Russian-made Sputnik V vaccine.
Limited vaccine availability threatens success
Even today, the stocks of COVID-19 vaccines remain low in most places, making the rollout slower than required for optimal protection and the prevention of vaccine-resistant variants. The situation is still worse in low-resource countries.
The Argentinian regulatory authority has authorized two vaccines as of now, and both developed on adenovirus viral vector platforms. These are the Gam-COVID-Vac (SPUTNIK V) and Oxford-AstraZeneca, with two doses being recommended for both of them.
Effect of pre-existing immunity on vaccination results
With the results of immunological research on recovered COVID-19 patients now being available, both humoral and cellular immunity has been found to persist for months following infection. This has raised the question of whether two doses of mRNA and adenovirus vaccines are needed in such patients as in naïve individuals.
The current study aims to examine the antibody response to the Sputnik V vaccine in a group of seropositive individuals for the virus compared to an uninfected cohort.
Study details
The participants consisted of volunteers from healthcare workers who received the vaccine between December 2020 and February 2021. The immunoglobulin G (IgG) antibody status following vaccination was examined using a high-quality enzyme-linked immunosorbent assay (ELISA) that targeted the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Antibodies to this protein have been shown to correlate well with neutralizing activity.
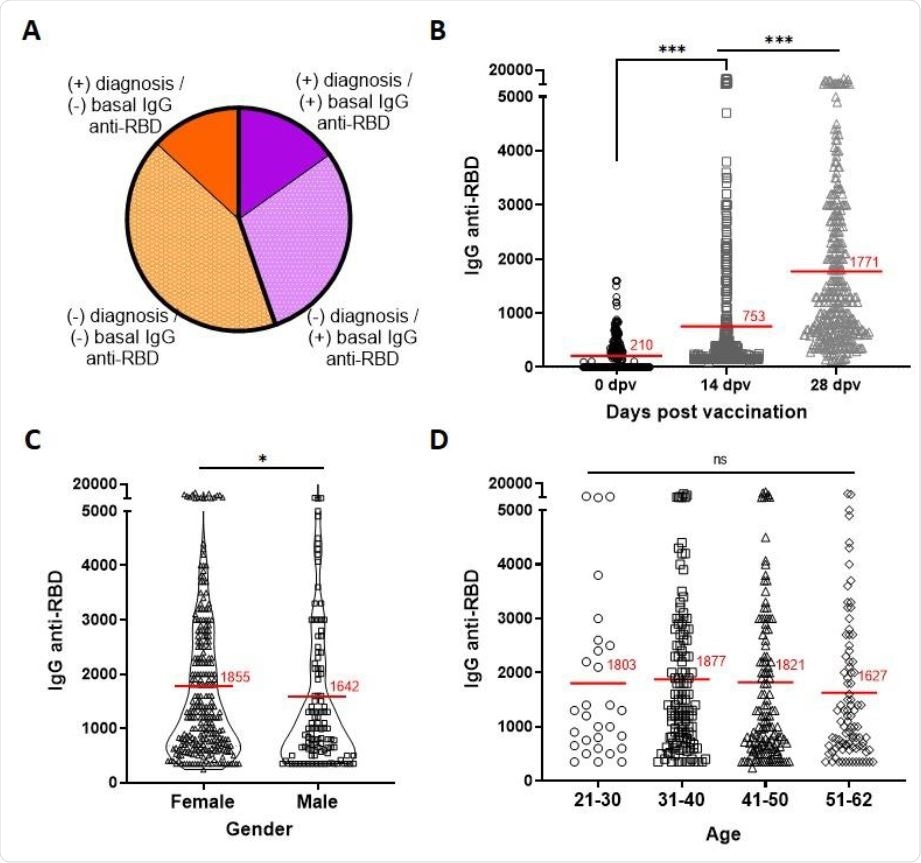
The baseline antibody titers directed against the RBD showed that 45% of the HCWs, overall, were seropositive, even though only 21% had a documented history of SARS-CoV-2 infection, as defined by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) or rapid antigen test.
What were the results?
Post-vaccination RBD-directed antibody titers were assessed on the day of vaccination and 14 and 28 days thereafter. The average titer of anti-RBD antibodies was 210 at day 0, but increased to 753 and 1771 on day 14 and day 28, respectively.
Seroconversion occurred in 97% of individuals following vaccination, with males and females showing a slight difference in the 28-day antibody titer. No age-based differences in titer were visible, however.
Seropositivity enhances immune response to vaccination
A subset of vaccine recipients was followed up to observe the longitudinal trend of antibody titers within the same individuals. Of this group, half received the second dose.
At 14 days from the first dose, both naïve and previously infected individuals showed higher antibody titers, but the titer was much higher in the seropositive group. This indicates that one dose of vaccine in this cohort fulfills the function of the booster dose, eliciting secondary rather than primary immunity.
This was confirmed in individuals who were thought to have had an asymptomatic infection, with the post-priming dose antibody titer on day 14 being 5.5-fold higher than in the seronegative cohort. It is notable that the asymptomatic-infection group had significantly higher baseline antibody titers relative to the controls, though the increase was slight.
In the group that received two doses of the vaccine, the seropositive cohort showed higher antibody responses than the seronegative group, at ~2,300 and 1,300, respectively. The response after the first dose among seropositive individuals on day 14 was higher, in fact, than that elicited by two doses of the vaccine in the seronegative cohort, on day 28.
Baseline antibody titer influences post-vaccination titer
It is noteworthy that the antibody response within the seropositive group is very heterogeneous, with the median titer being less than the average. Most individuals in this group who had a baseline anti-RBD IgG titer above 400 showed an impressive increase after one dose of the vaccine, compared to those with lower baseline titers.
This is despite the overall significant rise in anti-RBD titers following both the first and the second doses, indicating vaccine efficacy. This implies that “that the anti-RBD IgG titers at baseline have a direct consequence in the overall response to the vaccine.”
The study also showed that the duration since the onset of SARS-CoV-2 infection did not affect the eventual antibody titer following vaccination in seropositive individuals. Any duration from diagnosis, up to 120 days, seemed to elicit the same strong antibody response in this cohort.
What are the implications?
“In a context of limited access to SARS-CoV-2 vaccines, our findings show that in individuals previously exposed to the virus, the first dose of SPUTNIK V elicits a strong humoral immune response comparable or even higher than that reached after two doses in individuals without previous exposure to the virus.”
The effect of the intended prime vaccine dose in such individuals appears to be identical to that of the boost dose in a regular two-dose vaccine protocol, whether the initial infection was symptomatic or not. Thus, seropositive individuals with a baseline titer of above 400 could be assigned to receive a single dose without fear of eliciting an incomplete or non-protective immune response.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Chahla, R. E. et al. (2021). Past SARS-CoV-2 infection elicits a strong immune response after a single vaccine dose. medRxiv preprint. doi: https://doi.org/10.1101/2021.03.14.21253039. https://www.medrxiv.org/content/10.1101/2021.03.14.21253039v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News
Tags: Adenovirus, Antibodies, Antibody, Antigen, Assay, Conception, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Enzyme, Healthcare, Immune Response, Immunization, Immunoglobulin, Pandemic, Pathogen, Polymerase, Polymerase Chain Reaction, Protein, Receptor, Research, Respiratory, Reverse Transcriptase, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Viral Vector, Virus

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article