Alzheimers disease researchers extend the amyloid degradation toxicity hypothesis to the population level

Despite affecting millions worldwide, Alzheimer’s disease (AD) has long lacked effective treatments due to a fundamental inadequacy of our understanding of its etiology and pathogenesis. The absence of an integrative theory connecting the molecular origins of AD with disturbances at the organelle and cell levels, changes in relevant biomarkers, and population-level prevalence has hindered progress.
Even though most scientists only hope that an integrative theory of AD will emerge soon, scientists from Zarbio and Georgia State University discovered sufficient data to formulate a framework for such a theory.
The molecular and cellular levels of this framework were initially summarized as the amyloid degradation toxicity hypothesis. Diverging from previous amyloid-centric theories, it posits that beta-amyloid peptide (Aβ) enters cells through endocytosis and is transported to lysosomes for degradation. However, instead of complete degradation, some Aβ fragments form pores in lysosomal membranes, leading to cell death as lysosomal proteases leak into the cytoplasm.
The amyloid degradation toxicity hypothesis was recently extended to the population level in an article titled “Towards the Integrative Theory of Alzheimer’s Disease: Linking Molecular Mechanisms of Neurotoxicity, Beta-Amyloid Biomarkers, and Diagnosis.” Authored by Yaroslav Molkov (GSU), Maria Zaretskaia, and Dmitry Zaretsky (Zarbio), the article was published in the Current Alzheimer Research journal.
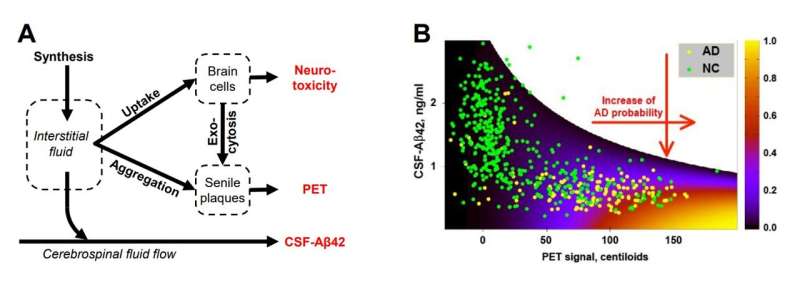
One of the central paradoxes in Alzheimer’s research is the strong correlation between AD diagnosis and the high density of non-toxic amyloid deposits (senile plaques) in the brain. The density can be measured by positron emission tomography (PET) and is an established AD biomarker.
The second paradox involves lower concentrations of toxic soluble Aβ42 in the cerebrospinal fluid (CSF-Aβ42, another AD biomarker) in AD patients. Any viable hypothesis must explain these phenomena, and the manuscript does that.
According to the amyloid degradation toxicity hypothesis, cytotoxicity depends on the cellular uptake of soluble Aβ rather than the presence of amyloid aggregates. The accumulation of cellular damage defines the probability of an AD diagnosis. Also, the authors argue that spontaneous extracellular seed formation is improbable due to low Aβ concentration.
In contrast, endocytosed Aβ is concentrated and stored intra-lysosomally, where it forms aggregation seeds. Aggregation seeds cannot be digested and, when exocytosed, grow into senile plaques. Therefore, to become aggregated, Aβ needs to be taken by brain cells first. The dependence of both Aβ toxicity and aggregation on the same process—cellular uptake of Aβ—resolves both paradoxes.
To test the validity of their conclusions, the researchers used the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database, the most comprehensive dataset on amyloid biomarkers. The model that formalizes the described concept reproduced clinical data at a 95% confidence level, supporting the amyloid degradation toxicity hypothesis. Notably, natural limitations on rates of cellular amyloid uptake explain the characteristic distribution of two amyloid biomarkers in the population.
“To the best of our knowledge, it is the first mechanistic explanation of why amyloid biomarkers are informative in AD diagnostics, even though they are indirectly related to the pathophysiology of the disease. Importantly, the amyloid degradation toxicity hypothesis not only addresses long-standing paradoxes but also provides a comprehensive framework that allows us to propose new pathophysiology-relevant biomarkers to diagnose or even predict AD,” says Dmitry Zaretsky, the Founder of Zarbio.
“Our next goal is to use the proposed framework to identify pharmacological targets that are scientifically relevant to AD pathophysiology.”
The findings from Zarbio and Georgia State University offer a promising step forward in understanding AD. They could pave the way for more effective treatments and preventive measures for this debilitating disease.
Source: Read Full Article