Analysis finds additional clinical benefits of molnupiravir for nonhospitalized participants with COVID-19

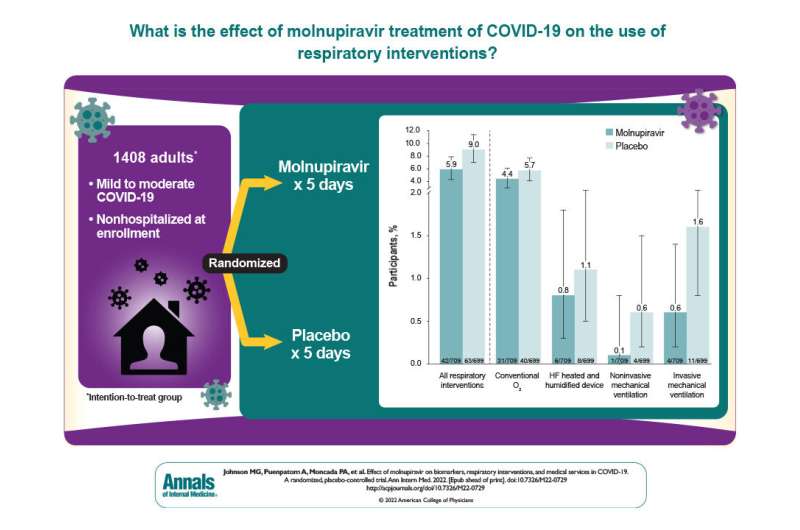
A secondary analysis of the randomized controlled MOVe-OUT trial found additional clinical benefits of molnupiravir for nonhospitalized participants with mild to moderate COVID-19 and risk factors for progression to severe disease. Participants treated with molnupiravir had a decreased need for respiratory interventions and fewer COVID-19-related acute care visits compared to those in the placebo group through day 29. The findings are published in Annals of Internal Medicine.
The phase 3 component of the MOVe-OUT randomized, controlled, clinical trial demonstrated the efficacy and safety of the oral antiviral, molnupiravir, for preventing hospitalization or death in high-risk nonhospitalized participants with mild to moderate COVID-19 through day 29. Participants who received molnupiravir showed a shorter time to resolution for most COVID-19 signs and symptoms, a greater reduction in mean viral load from baseline, and a lack of safety concerns compared with placebo. Additional clinical benefits of molnupiravir were not analyzed at that time.
The researchers conducted a secondary analysis of the MOVe-OUT trial to evaluate additional potential benefits of molnupiravir for the treatment of mild to moderate COVID-19 based on clinical markers and the need for respiratory interventions and medical services. Changes in high-sensitivity C-reactive protein (CRP) concentration and Spo2, and the need for respiratory interventions, acute care visits, and COVID-19–related acute care visits were evaluated in participants who received molnupiravir or placebo.
Source: Read Full Article