Deciphering the secrets of spinal cord regeneration protein by protein
In mammals, including humans, scar tissue forms after injury to the spinal cord as part of the healing process. In mammals, however, this has a serious drawback: the scar tissue cannot be penetrated by regrowing nerves. As a result, severed nerves cannot regenerate. In the case of spinal cord injury, this leads to permanent paralysis.
In previous studies, Daniel Wehner showed that zebrafish also form scar-like wound tissue after spinal cord injury. However, zebrafish are able to regenerate nerves and regain motor function, even after severe spinal cord injury. How they are able to do this is still poorly understood. Wehner and his team at the Max Planck Institute for the Science of Light (MPL) are trying to change that.
Zebrafish, the subject of Wehner’s research, are frugal and undemanding. As vertebrates, they are genetically similar to humans; more than 80 percent of the genes known to cause human diseases are also found in these fish. In addition, zebrafish larvae are transparent, which means that tissue formation can be studied in the living animal using state-of-the-art imaging techniques, some of which are being developed at the MPL.
In previous scientific work, Wehner’s research group and scientists from the Department of Biological Optomechanics led by Jochen Guck, director at the institute in Erlangen, have already shown that there “must” be differences between the biochemical composition and the mechanical properties of scar tissue in mammals and wound tissue in zebrafish. In zebrafish, spinal cord tissue stiffens after injury, while the opposite is observed in mammals.
In addition, the scientists found that nerve fibers in zebrafish can not only grow through the wound tissue, but their growth is actually stimulated by it. In a new paper published in the journal Nature Communications, the team has now identified another piece of the puzzle in understanding the difference between wound healing and the associated ability of the spinal cord to regenerate in mammals and zebrafish.
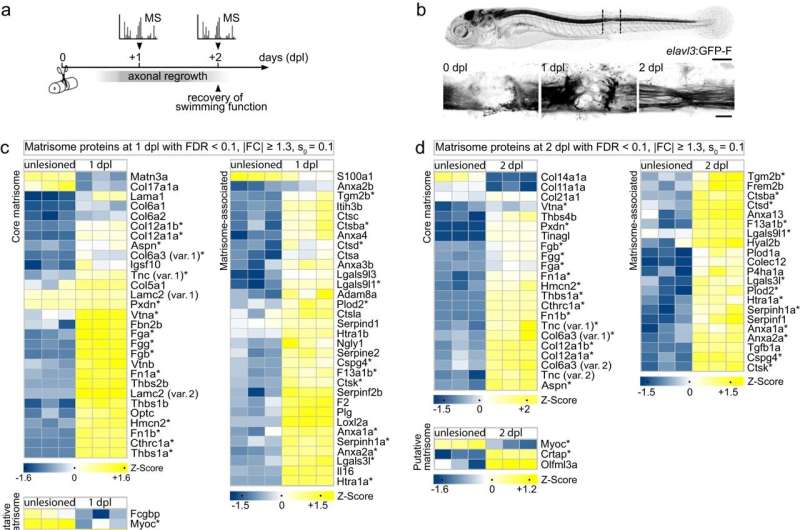
In the current study, Wehner and his colleagues compared rat wound tissue with that of zebrafish to find new components that might interfere with nerve regeneration in mammals.
“We wanted to find out if there are inhibitory proteins in rat scar tissue that are not present in zebrafish,” explains Julia Kolb, first author of the publication and a Ph.D. student in Wehner’s group.
In an interdisciplinary collaboration between the MPL research groups of Wehner, Kanwarpal Singh, and the department of director Guck, the scientists were able to identify proteins belonging to the family of small leucine-rich proteoglycans (SLRPs), to be highly abundant in the scar tissue of rats, mice and humans. However, they were barely detectable in the wound tissue following spinal cord injury in zebrafish.
The team then used state-of-the-art genetics to to increase the abundance of SLRP proteins in the zebrafish wound tissue. The result was clear: The regenerative capacity of the manipulated fish was significantly reduced and the mechanical properties of the wound tissue changed to a state similar to that of mammalian scar tissue.

Wehner says, “This result is not only extremely exciting and offers an explanation for the difference in regenerative capacity between humans and zebrafish, but also opens up the possibility that we can gradually better understand the development of scar tissue in mammals. The zebrafish lacks many factors that inhibit nerve regeneration in mammals. Such components, such as the SLRPs studied by the MPL group, can be isolated and individually tested for their mode of action in fish.”
“With this understanding, we hope to develop new therapeutic approaches for spinal cord injury in humans in the future.”
More information:
Julia Kolb et al, Small leucine-rich proteoglycans inhibit CNS regeneration by modifying the structural and mechanical properties of the lesion environment, Nature Communications (2023). DOI: 10.1038/s41467-023-42339-7
Journal information:
Nature Communications
Source: Read Full Article