Genetic switches in tumor development may lead to new therapeutic target for colorectal cancer
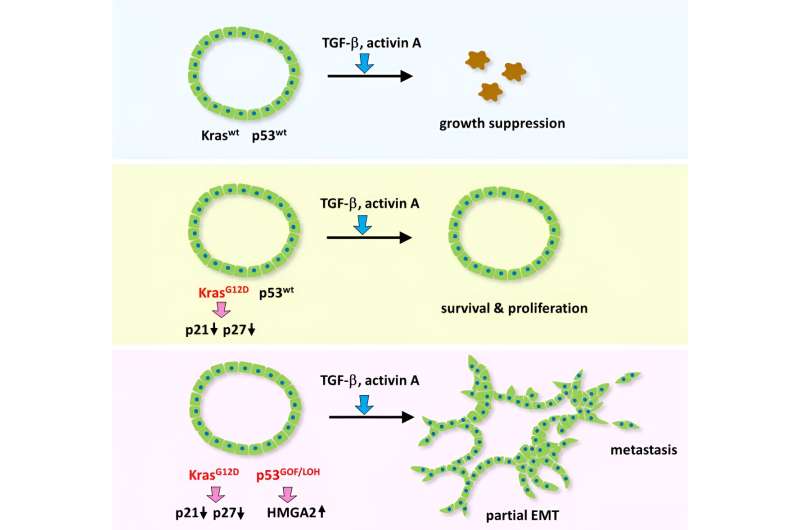
Researchers at Kanazawa University report in Cancer Research how Kras and p53 mutations influence the tumor suppressor and promoter functions of a TGF- ß pathway. The findings may lead to a new approach for colorectal cancer therapy.
Both the progression and the suppression of tumors are governed by biomolecular processes. Often, a particular process is involved in either cancer progression or suppression. Cancer treatment in the form of drugs then typically focuses on the respective deactivation or activation of the relevant biomolecular process. However, it has been established that a process known as transforming growth factor ß (TGF-ß) signaling) plays a role in both tumor suppression and progression.
Now, Masanobu Oshima from Nano Life Science Institute (WPI-NanoLSI), Kanazawa University and colleagues have studied the precise genetic conditions underlying the outcome of TGF-ß signaling. Their findings may help the development of new therapeutic strategies for particular cancers.
The suppressive effect of TGF-ß signaling happens through the stimulation of cell differentiation—the process through which dividing cells acquire their type or function. The malignant progression of cancers, on the other hand, comes from a process called epithelial-mesenchymal transition (EMT), in which an epithelial cell transforms into a mesenchymal cell type. The former is a ‘stationary’ type of cell, found in epithelial tissue, whereas the latter is a more ‘migratory’ type of cell found in development and cancer.
Oshima and colleagues performed experiments with tumor-derived organoids. They confirmed that TGF-ß family cytokine, activin plays a role in tumor suppression and progression dependent on the mutation types of driver genes.
In certain cancer cells treated with activin, the researchers noted that the partial EMT is induced with tumor aggressiveness and development. On the other hand, certain mutated activin receptors were found to have cancer suppressor capabilities, which made the scientists conclude that genetic alterations underlie the dual function of activins.
One of the two relevant genes is Kras which relays signals that regulate cell growth, division and differentiation. Oshima and colleagues found that a mutation of Kras blocks TGF-ß/activin-induced growth suppression. The other gene is known as Trp53, which encodes tumor protein 53, playing an important role in cancer regulation.
A combination of Kras and Trp53 mutations at hot spots, known as gain-of-function mutation, was found to not just block tumor suppression but promote partial EMT and tumor proliferation.
The experiments were conducted using mouse intestinal tumor-derived organoids with defined genetic backgrounds, which makes the results relevant for therapeutic strategies for human colorectal cancer.
“Based on these results, the control of TGF- ß/activin signaling appears to be an important preventive and therapeutic strategy against the malignant progression of colorectal cancer carrying […] mutations,” the scientists say.
More information:
Dong Wang et al, Gain-of-function p53 mutation acts as a genetic switch for TGF-β signaling-induced epithelial-to-mesenchymal transition in intestinal tumors, Cancer Research (2023). DOI: 10.1158/0008-5472.CAN-23-1490
Journal information:
Cancer Research
Source: Read Full Article