Harnessing the power of a parasite that can stop pain
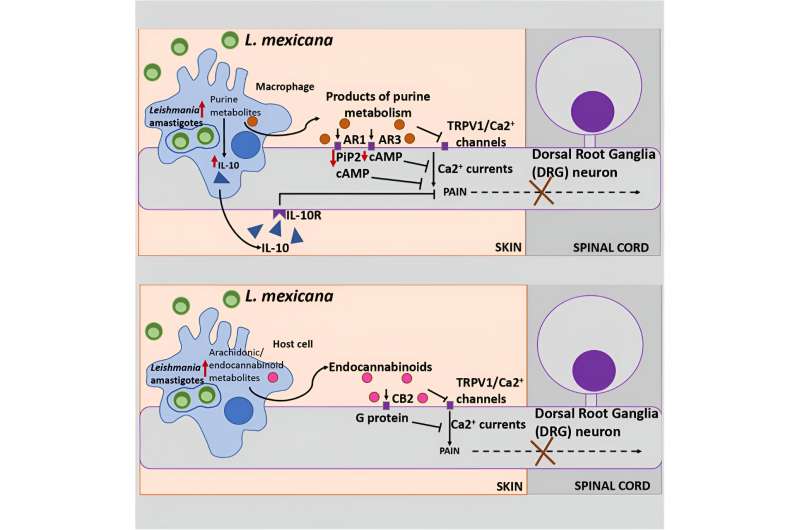
For the first time, scientists have begun to figure out why the disfiguring skin lesions caused by cutaneous leishmaniasis don’t hurt.
Researchers analyzed leishmaniasis lesions on mouse skin to detect metabolic signaling pathways that differed from uninfected mice. Results suggested the parasites that cause the disease change pain perception—presumably as a way to delay treatment and promote their own survival.
“No one knows why these lesions are painless—but it has been thought that the parasite somehow manipulates the host physiological system,” said Abhay Satoskar, senior author of the study and professor of pathology at The Ohio State University College of Medicine.
“Based on our data, something the parasites do triggers pathways that suppress pain. How they do that, we’re still investigating.”
Beyond increasing understanding of this parasitic disease afflicting 1 million new patients each year, the research could lead to the development of new non-narcotic pain medications.
“We hypothesize that any molecules the parasite’s presence is producing could be potential painkillers for other health problems,” Satoskar said.
The study was published recently in the journal iScience.
The lack of pain in leishmaniasis lesions has puzzled scientists for years, especially when similar blisters caused by conditions like chicken pox, staphylococcus infections, or the herpes virus are itchy, oozy, and sore.
After giving mice chronic infections with Leishmania mexicana, the species that causes cutaneous leishmaniasis in South, Central, and North America, researchers used an unbiased mass spectrometry analysis of the lesions to identify molecules known to be associated with pain suppression.
They found numerous metabolites—products of biochemical reactions that break down food to produce energy and perform other essential functions—that have been linked in previous research to blockage of pain perception. They also found pathways with pain-relief properties tied to the brain’s endocannabinoid system, which involves a host of physiological processes, including the pain response.
Cell-culture experiments in infected macrophages, the immune cells in which Leishmania parasites live, showed an increase in most, but not all, of the same changes as in the lesions.
The parasites use these metabolites as nutrition to help them replicate. But finding that specific pain-suppression pathways aren’t increased in infected macrophages leaves some questions unanswered, said Satoskar, also a professor of microbiology at Ohio State.
“The infection does something in the cell that could be a direct or indirect effect—we don’t know. But the environment that the infection creates leads to production of these metabolites,” he said. “The exciting thing is that this is the first time we’ve begun to understand the cellular basis of why there is no pain in these lesions.”
“The next key question is, if we know these pathways are responsible, then how are they triggered? By the parasite, or something the parasite is doing to the host cell, or a combination of both? There could be a lot of things happening.”
Skin test for immunity to Leishmania-caused diseases
Satoskar has also co-led an initiative to develop a standardized skin test to check for immunity to Leishmania donovani, the parasite that causes visceral leishmaniasis—a potentially lethal form of the disease that affects the organs and is fatal if untreated.
He and colleagues recently reported in Nature Communications on this work, which is critical for disease surveillance in the most affected regions of the world and will be needed for phase 3 clinical trials of leishmaniasis vaccines the team has developed.
The test, using an antigen called leishmanin, is similar to a skin test for tuberculosis—a positive response means a person has been exposed to the parasite and has cellular immunity that prevents further clinical symptoms.
No tests or reagents are available to detect sporadic cases of leishmaniasis reported in the southern United States, so the skin test being developed will facilitate surveillance studies to assess exposure throughout the endemic regions for leishmaniasis—which include the U.S.
“This is a very important test in the field to understand who is exposed to this disease or not,” Satoskar said. “For many stakeholders going into a community to conduct surveillance, knowing who is immune and who is not immune is essential so they can deploy their limited resources appropriately for disease control.”
Leishmanin skin tests have existed and been used in past years but are no longer available. This research team developed the antigen following Good Laboratory Practice guidelines and tested it in hamsters, a model for human visceral leishmaniasis, to ensure the skin test triggers the expected immune response to both infection and vaccination.
“This kind of data can be used for fast-track approval for Leishmania vaccines, which are under development. We anticipate fulfilling the need of the entire global community,” Satoskar said.
More information:
Greta Volpedo et al, Leishmania mexicana Promotes Pain-reducing Metabolomic Reprogramming In Cutaneous Lesions, iScience (2023). DOI: 10.1016/j.isci.2023.108502
Ranadhir Dey et al, Production of leishmanin skin test antigen from Leishmania donovani for future reintroduction in the field, Nature Communications (2023). DOI: 10.1038/s41467-023-42732-2
Journal information:
Nature Communications
,
iScience
Source: Read Full Article