How novel pathogens may cause the development of colorectal cancer

Do BMMFs, the novel infectious agents found in dairy products and bovine sera, play a role in the development of colorectal cancer? Scientists led by Harald zur Hausen detected the pathogens in colorectal cancer patients in close proximity to tumors. The researchers show that the BMMFs trigger local chronic inflammation, which can cause mutations via activated oxygen molecules and thus promote cancer development in the long term. BMMFs and inflammatory markers were significantly more frequently detectable in the vicinity of malignant intestinal tumors than in the intestinal tissue of tumor-free individuals.
A few years ago, scientists led by Ethel-Michele de Villiers at the German Cancer Research Center (DKFZ) discovered a novel form of infectious agents in dairy products and bovine sera. These were ring-shaped DNA elements that showed great similarity to sequences of certain bacterial plasmids. They were named Bovine Meat and Milk Factors (BMMFs) after their origin in bovine products.
De Villiers, together with Harald zur Hausen, had tracked down the infectious agents while testing a hypothesis: Based on epidemiological observations, the Nobel Prize winner zur Hausen had postulated a possible link between the consumption of beef or dairy products and the incidence of colorectal cancer. “It seemed likely to us that an infectious agent transmitted from European domestic cattle to humans was associated with the development of colorectal cancer,” zur Hausen said.
Meanwhile, de Villiers was able to isolate more than a hundred different agents from dairy products. The BMMFs can multiply in human cells, where they produce a protein product, Rep, which they need to multiply. But how might they contribute to the development of colorectal cancer?
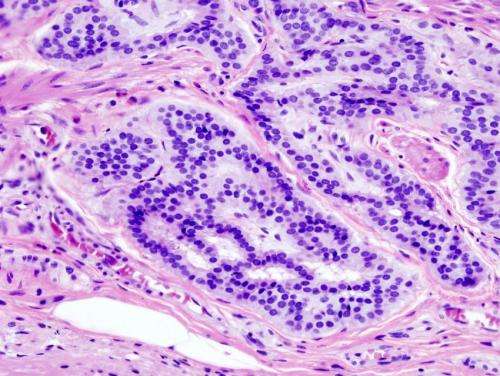
Scientists from Timo Bund’s team at the DKFZ have now carefully investigated this question using tissue samples from colorectal cancer and from healthy intestine. To detect the pathogens, the researchers used antibodies generated against the Rep protein. This enabled them to detect BMMFs in 15 of 16 colorectal cancer tissue samples.
To the surprise of the scientists, staining tissue sections using these antibodies revealed: Not the cancer cells themselves contained the Rep protein, but the cells in the immediate vicinity of the tumors. In particular, the antibody detected the Rep protein in the lamina propria, the connective tissue layer located under the intestinal mucosa, and there especially in the vicinity of the intestinal crypts. From these Rep-positive cells, the researchers were also able to isolate BMMF DNA that was closely related to the pathogens already isolated from milk samples.
The research team suspected that the presence of BMMFs could trigger chronic inflammatory processes in intestinal tissue. One indication of an inflammation would be the presence of pro-inflammatory macrophages. Indeed, these inflammatory cells were found in the immediate vicinity of the tumors. Interestingly, the signals for the Rep protein and for the macrophage marker CD68 were almost congruent: Rep is thus present immediately around or in the macrophages.
But is the presence of BMMFs and the associated chronic inflammation really associated with colorectal cancer? To find out, Bund and colleagues looked for combined Rep/CD68 signaling in colorectal cancer samples and compared them with colorectal tissue samples from a group of younger cancer-free control subjects. In the cancer patients, 7.3 percent of all intestinal cells in the tumor environment were positive for combined Rep/CD68 signals. In the intestinal cells of the control group, this figure was significantly lower at only 1.7 percent.
Another indication of inflammatory processes were the elevated levels of reactive oxygen species that Ethel-Michele de Villiers, Timo Bund and colleagues detected in the environment of Rep-positive cells. “Such oxygen radicals promote the development of genetic mutations,” explains Harald zur Hausen. The inflammations were particularly localized in the immediate vicinity of the intestinal crypts. These tubular cavities are home to the intestinal stem cells, which are responsible for the constant regeneration of the intestinal mucosa. Intestinal stem cells continuously produce large quantities of progenitor cells, which divide rapidly and are exposed to this mutation-promoting influence. The more mutations accumulate, the higher the risk that genes will also be hit that will cause cell growth to spiral out of control. Chronic inflammation is known to drive cancer, a well-known example being the development of liver cancer as a result of chronic infection with the hepatitis C virus.
“We therefore regard BMMFs as indirect carcinogens, which probably act on the dividing cells of the intestinal mucosa for decades,” zur Hausen said. He hypothesizes that infection with BMMFs usually occurs early in life, around the time of weaning.
Source: Read Full Article