New immunotherapy treatment targets respiratory viral infections
A University of Central Florida College of Medicine researcher has developed a new, more precise treatment for a major cause of illness around the world each year—acute respiratory viral infections.
Acute respiratory viral infections include sicknesses such as the flu, pneumonia, respiratory syncytial virus (RSV) and coronavirus. These infections cause millions of illnesses worldwide, with the flu alone responsible for 3 million to 5 million cases of severe illness and up to 650,000 respiratory deaths each year, according to the World Health Organization.
The new treatment, which is now available for licensing, uses immune system-related proteins known as cytokines in a controlled, targeted manner to fight infections and promote healing.
“Interventions that can improve the outcome of serious viral infection without interfering with effective anti-viral immune responses are urgently needed,” says Tara Strutt, an assistant professor in the Burnett School of Biomedical Sciences within UCF’s College of Medicine, who developed the treatment.
As a cellular immunologist, Strutt knows very well what cytokines—part of the body’s defense against pathogens—can do.
“Cytokines can do good things, and they can do bad things,” she says. “For example, during severe infections, an overactive immune response can trigger a cytokine storm, a condition in which excess cytokine production leads to uncontrolled inflammation. This hyperinflammation can ultimately cause tissue damage and organ failure.”
The cytokine Strutt is using, interleukin-2 (IL-2), is currently approved for cancer immunotherapy. One of the challenges of IL-2-based therapy, though, is the numerous off-target effects that can result in organ and tissue damage, Strutt says.
To overcome this, Strutt’s immunotherapy treatment uses IL-2 combined with a monoclonal antibody specific for IL-2 to form an IL-2 antibody complex, or targeted IL-2. The targeted IL-2 can induce both anti-inflammatory and pro-inflammatory responses. By modulating inflammatory response, further complications such as cytokine storms can be avoided and clinical outcomes can be improved.
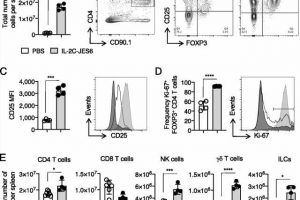
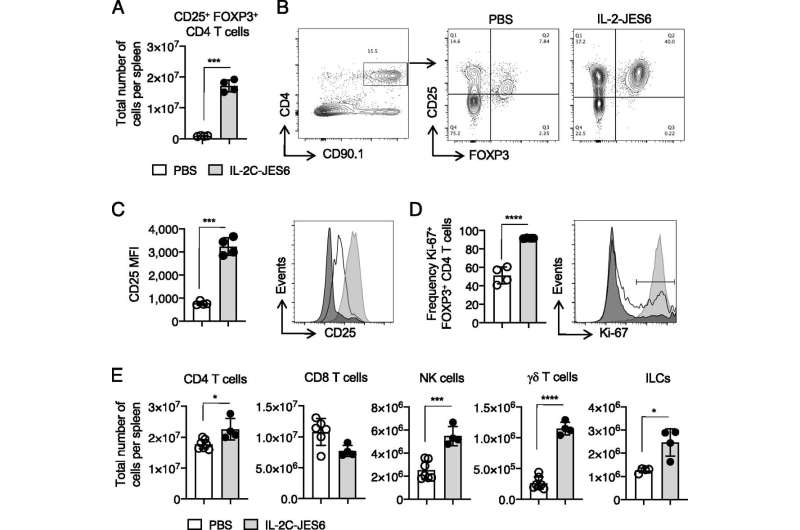
In animal studies done in the Strutt laboratory, improved outcomes—including improved respiratory function and reduced airway inflammation—were seen when targeted IL-2 was administered during influenza virus infection. Targeted IL-2 was shown to promote an antiviral inflammatory response during viral infection and reduced—virtually absent—bronchial inflammation.
Additionally, the use of the targeted IL-2 was shown to have a beneficial role in forming memory CD4 T-cells. These CD4 T-cells protect against pathogens previously encountered by infection or vaccination. This application has the potential to improve the immune responses stimulated by vaccines, Strutt says.
At UCF, Strutt is studying CD4 T-cells and how they function to protect against respiratory viruses, including Influenza A virus (IAV). The researcher and her team are also exploring how memory T cell responses function during pregnancy, as pregnant females are at high risk for serious IAV infection associated complications.
“CD4 T-cells are the orchestrators of the immune response,” she says.
Also known as helper T-cells, CD4 T-cells produce and secrete specific cytokines that act as immune system messengers. During an IAV infection, subsets of CD4 T-cells become activated and start to produce and secrete IL-2, a potent T-cell growth factor with both proinflammatory and anti-inflammatory effects.
Before joining UCF in 2015, Strutt was an assistant professor at the University of Massachusetts Medical School. She completed her postdoctoral work at the Trudeau Institute in New York and received her doctorate in immunology from the University of Saskatchewan.
Her work strives to provide greater insight for the development of safe, innovative vaccine formulations and immune-based therapies for respiratory viral infections.
Journal information:
Journal of Immunology
Source: Read Full Article