Researchers report ‘hybrid immune damping’ following SARS-CoV-2 Omicron infection
A recent Science journal study explores the impact of differential immune imprinting patterns in individuals vaccinated against the coronavirus disease 2019 (COVID-19) with a history of prior infection with a variant of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the Omicron wave.

Study: Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Image Credit: Starshaker / Shutterstock.com
Background
By late 2021, the SARS-CoV-2 Delta variant of concern (VOC) was replaced by the Omicron VOC. The SARS-CoV-2 Omicron variant possesses several spike (S) mutations that contribute to its increased transmissibility and partial evasion from neutralizing antibodies (nAbs).
Previous evidence has shown that the Omicron variant and its sublineages have reduced the efficacy of mitigation strategies to contain the COVID-19 pandemic. Current COVID-19 vaccines remain protective against severe illness and mortality; however, they are limited in their ability to prevent breakthrough infections. This has been demonstrated by reports from nations with high COVID-19 vaccination rates that are currently experiencing frequent reinfections and breakthrough infections with circulating Omicron variants.
About the study
The present study explored T- and B-cell immunity toward the Omicron variant among healthcare workers (HCWs) with various types of SARS-CoV-2 infection and/or vaccination histories to determine the effect of immune imprinting.
To this end, HCWs who experienced mild and asymptomatic SARS-CoV-2 infection during the initial COVID-19 wave caused by the Wuhan Hu-1 ancestral strain, as well as those infected when the Alpha, Delta, and Omicron VOCs were the dominant circulating strains, were included in the current study. HCWs with and without a history of COVID-19 who were triple vaccinated with the Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) COVID-19 vaccine were also included in the current study.
T-cell reactions against the naturally processed antigen and peptide pools were assessed, in addition to memory B-cell (MBC) frequency, S subunit 1 (S1) receptor binding domain (RBD) and full S binding, and live virus nAb effectiveness.
Study findings
Regardless of their infection history, nAb responses eventually plateaued in HCWs after receiving three COVID-19 vaccine doses. To this end, HCWs previously infected during the initial COVID-19 wave caused by the ancestral Wuhan Hu-1 strain exhibited significantly reduced anti-RBD titers against the Beta, Gamma, and Omicron variants as compared to infection-naïve HCWs who received three COVID-19 vaccine doses.
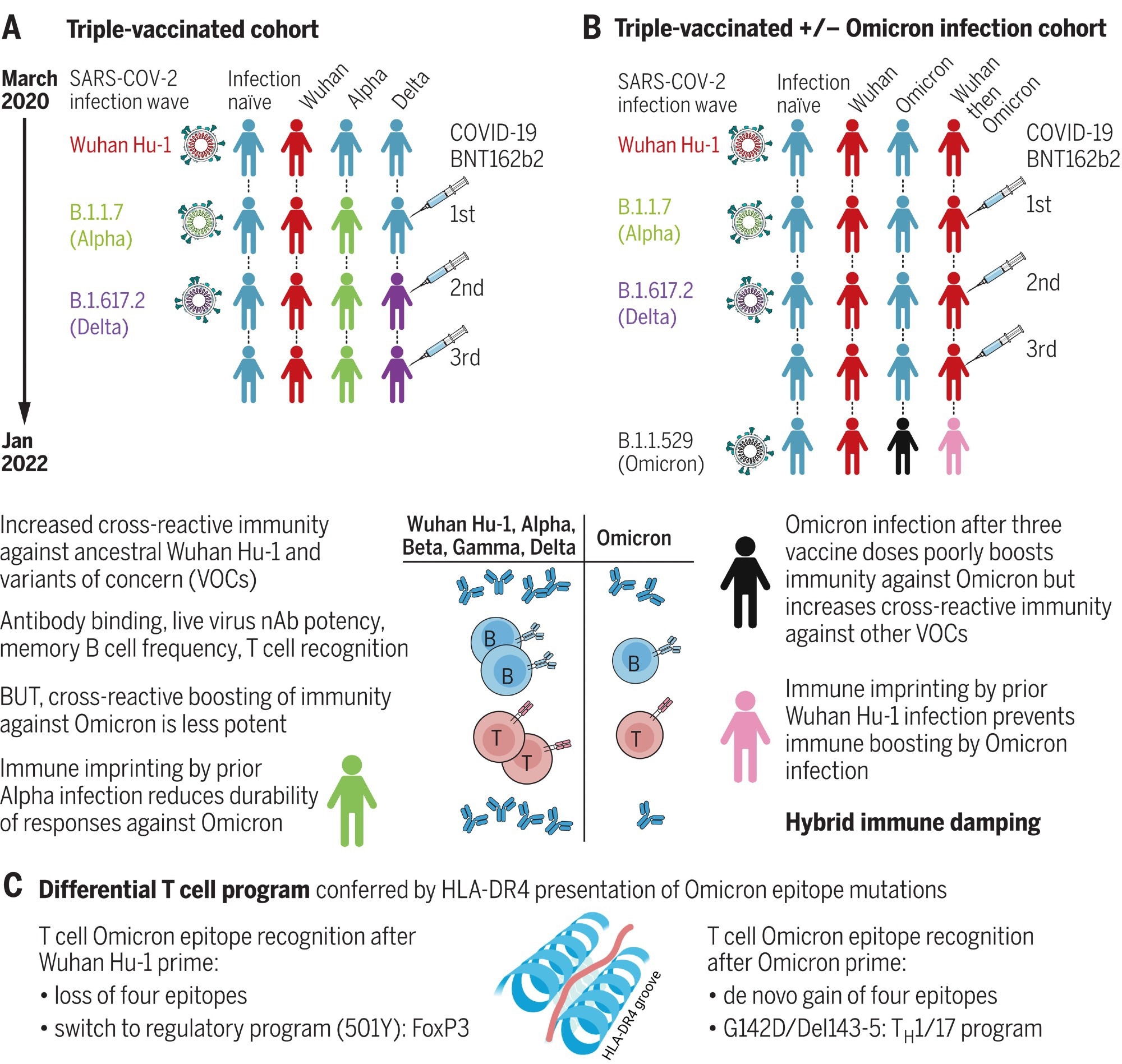 Hybrid immune damping. (A) Triple-vaccinated HCWs with different SARS-CoV-2 infection histories show boosted cross-reactive immunity against VOCs, less so against Omicron. (B) Breakthrough infection during the Omicron wave boosts cross-reactive immunity in triple-vaccinated, previously infection-naïve individuals against VOCs, less so against Omicron itself; imprinting by previous Wuhan Hu-1 infection ablates Omicron immune boosting. (C) T cell recognition of Omicron mutation sequences is linked to altered transcription.
Hybrid immune damping. (A) Triple-vaccinated HCWs with different SARS-CoV-2 infection histories show boosted cross-reactive immunity against VOCs, less so against Omicron. (B) Breakthrough infection during the Omicron wave boosts cross-reactive immunity in triple-vaccinated, previously infection-naïve individuals against VOCs, less so against Omicron itself; imprinting by previous Wuhan Hu-1 infection ablates Omicron immune boosting. (C) T cell recognition of Omicron mutation sequences is linked to altered transcription.
Comparatively, HCWs who were previously infected with Wuhan Hu-1 and Alpha strains exhibited more potent nAb responses against the Wuhan Hu-1, Alpha, and Delta variants. Notably, a history of prior SARS-CoV-2 infection did not improve cross-reactive S1 RBD IgG titers against the Omicron variant.
In terms of T-cell responses, the researchers observed that regardless of previous SARS-CoV-2 infection history, T-cell responses were reduced against the Omicron variant.
Triple vaccinated HCWs who were previously infected with the Wuhan Hu-1 strain and subsequently infected with the Omicron variant exhibited the highest nucleocapsid nAb binding as compared to any other prior SARS-CoV-2 variant infection. Even infection-naïve triple vaccinated HCWs who were subsequently infected with the Omicron variant produced significantly increased cross-reactive nAb responses against all VOCs.
Omicron infection did not produce the same extent of cross-reactive antibody immunity to the Omicron variant. Furthermore, infection-naïve triple vaccinated HCWs exhibited rapid nAb waning following their third dose against the Omicron variant, as demonstrated by the lack of nAbs produced 14 weeks after their third dose against the Omicron variant.
Interestingly, any protection that was conferred against the Omicron variant in previously naïve HCWs who were infected with Omicron was not observed in HCWs previously infected with the Wuhan Hu-1 strain and subsequently reinfected with the Omicron variant.
As compared to these reduced B-cell responses, previous infection with Wuhan Hu-1 and Delta strains provided a greater level of T-cell immunity against the Omicron variant as compared to HCWs who were previously infection-naïve.
Omicron infection did not produce cross-reactive T-cell responses against the Omicron variant; however, these responses were observed against other SARS-CoV-2 variants. However, prior infection with the Wuhan H-1 and Alpha variants in unvaccinated individuals did not produce any cross-reactive S1 RBD antibodies against the Omicron variant.
Conclusions
Despite reduced neutralization by vaccination and prior infection, three doses of COVID-19 mRNA vaccine provide 50-70% protection against symptomatic disease. This protection is believed to be due to the maintenance of relatively high T-cell responses that are not altered by reduced nAb levels.
The current study demonstrated that prior infection with both Wuhan Hu-1 and Alpha variants in vaccinated individuals actually has a negative effect on their subsequent protective immunity against Omicron infection. These findings support a process known as “hybrid immune damping,” wherein specific imprinted patterns of vaccination and infection reduce protection against future infection.
The researchers also demonstrated that Omicron infection confers poor immunogenicity against itself, thus explaining why frequent Omicron reinfections have been reported in short time intervals. This evidence also negates a potential benefit of future booster vaccine doses that are based on the Omicron sequence.
- Reynolds, C., Pade, C., Gibbons, J., et al. (2022). Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science 377(6603). doi:10.1126/science.abq1841.
Posted in: Medical Science News | Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, Antigen, Cell, Coronavirus, Coronavirus Disease COVID-19, covid-19, Efficacy, Frequency, Healthcare, immunity, Mortality, Omicron, Pandemic, Receptor, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, T-Cell, Vaccine, Virus

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article