SARS-CoV-2 antibodies in breast milk remain after Holder pasteurization

Many issues regarding the coronavirus disease 2019 (COVID-19), including its prognosis, management, and interactions with the immune system, as well as the evolution of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remain unanswered. One highly vulnerable patient population to the severe effects of COVID-19 includes pregnant mothers.

Study: Anti-SARS-CoV-2 IgA and IgG in Human Milk From Vaccinated Mothers After Holder Pasteurization. Image Credit: comzeal images / Shutterstock.com
Background
According to the World Health Organization (WHO), breastfeeding is considered the gold standard for feeding children until the age of two. Thus, mothers are often encouraged to continue this practice, particularly during the current COVID-19 pandemic, as long as their clinical status is allowed.
An infant’s health and development are aided by breast milk due to the presence of several beneficial biological components including hormones, immunoglobulins (Ig), cytokines, growth factors, and microorganisms. When breastfeeding is not available, two alternative food sources for infants include milk formula or donated and pasteurized human milk (DHM).
To prevent possible contamination from pathogenic organisms or agents, DHM is pasteurized in milk banks. Unfortunately, the pasteurization process can cause some of the biological, structural, and functional features of breast milk to be lost.
Previous studies have demonstrated the maternal-infant antibody transfer through breast milk after maternal recovery from COVID-19 and immunization. However, it remains uncertain whether these antibodies can survive pasteurization and continue to provide passive protection to the infant.
About the study
In a recent prospective and observational study under consideration at the International Breastfeeding Journal and currently posted to the Research Square* preprint server, the impact of pasteurization on specific Ig concentration against SARS-CoV-2 in milk from messenger ribonucleic acid (mRNA) vaccinated lactating women was evaluated.
Between January 2021 to April 2021, all participants were selected in the Autonomous Community of Valencia (Spain), and, as health care workers, they were assigned to vaccination priority groups. All lactating women were given two doses of the Pfizer/BioNTech mRNA BNT162b2 vaccine. Participants were also asked to provide further clinical and demographic information.
Antibody levels were measured before and after pasteurization. Enzyme-linked immunosorbent assay (ELISA) was utilized to determine antibodies directed to the receptor-binding domain (RBD) of SARS-CoV-2.
Before analysis, specimens were diluted 1:4 and, to aid in the determination of Ig levels, a standard curve was included. The standard curve was made up of ten three-fold serial dilutions of a mixture of materials that had previously been evaluated and exhibited elevated amounts of both Ig concentrations.
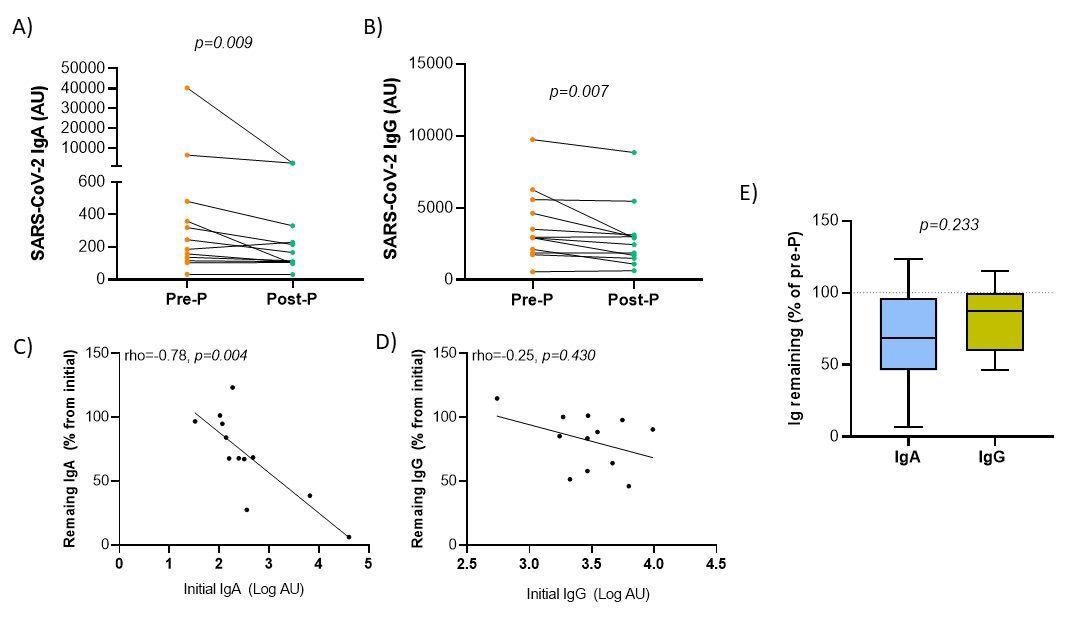
Variation of anti-SARS-CoV-2 antibody levels in breast milk after Holder pasteurization. Panels A-B. Comparison of immunoglobulin A (A) and G (B) antibody levels before (Pre-P) and after (Post-P) pasteurization. Panel C-D. Spearman’s rank correlation between the initial levels of Ig in log-transformed arbitrary units (AU) and the percentage of remaining Ig respecting the initial. Panel E. Comparison between the remaining immunoglobulin percentages after the pasteurization process according to immunoglobulin isotype. Wilcoxon matched-pairs signed-rank test was used to determine the significance of the difference between both isotypes.
Participant demographics and characteristics
The current study consisted of twelve lactating women with a mean age of 35 years, whereas the children of the participants had a mean age of 11 months. When their mothers received the first dose of the vaccine, the mean weight of the children was 8.5 kg. After the mothers were vaccinated against COVID-19, none of the children displayed signs of a fever and no recorded serious adverse events were recorded.
SARS-CoV-2 antibodies in breast milk after pasteurization
Following Holder pasteurization, both anti-SARS-Cov-2 isotypes IgA and IgG were significantly reduced. Both isotypes exhibited a significant negative correlation between the initial level of anti-SARS-CoV-2 antibodies and the percentage of their recovery following pasteurization.
Despite the partial reduction in IgA and IgG concentrations, a significant percentage of antibodies persisted following pasteurization. Following pasteurization, 70.53% of anti-SARS-CoV-2 IgA antibodies and 81.99% of IgG antibodies were observed. After pasteurization, the percentage of lost antibodies appeared to be lower when IgG levels were compared to IgA levels.
Implications
Although SARS-CoV-2 IgA and IgG levels in human milk were found to decrease after Holder pasteurization, a considerable percentage of antibodies were retained.
The persistence of antibodies in breast milk, even after pasteurization, supports providing breast milk to infants during the COVID-19 pandemic. Furthermore, the current findings emphasize the possible importance of virus-specific SARS-CoV-2 antibodies in providing passive protection in infants receiving breast milk. More research is needed to determine the efficacy of these antibodies and the duration of their protection in breastfed infants.
*Important notice
Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Selma-Royo, M., Gormaz, M., Bäuerl, C., et al. (2022). Anti-SARS-CoV-2 IgA and IgG in Human Milk From Vaccinated Mothers After Holder Pasteurization. Research Square. doi:10.21203/rs.3.rs-1292319/v2. https://www.researchsquare.com/article/rs-1292319/v2.
Posted in: Child Health News | Medical Procedure News | Medical Research News | Women's Health News | Disease/Infection News
Tags: Antibodies, Antibody, Assay, Breast Milk, Breastfeeding, Children, Contamination, Coronavirus, Coronavirus Disease COVID-19, covid-19, Cytokines, Efficacy, Enzyme, Evolution, Fever, Food, Health Care, Immune System, Immunization, Pandemic, Receptor, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus
.jpg)
Written by
Colin Lightfoot
Colin graduated from the University of Chester with a B.Sc. in Biomedical Science in 2020. Since completing his undergraduate degree, he worked for NHS England as an Associate Practitioner, responsible for testing inpatients for COVID-19 on admission.
Source: Read Full Article