Study identifies novel epigenetic changes in pediatric brain cancer
Investigators have identified previously unknown sets of epigenetic changes in pediatric brain tumors, which could serve as novel therapeutic targets and provide alternative treatment options for patients, according to a Northwestern Medicine study published in Nature Communications.
Xiao-Nan Li, MD, Ph.D., the Rachelle and Mark Gordon Professor of Cancer Research and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, was senior author of the study.
Pediatric ependymoma, the third most common type of brain cancer in children, currently has a recurrence rate of 50% and most patients will eventually succumb to the disease due to poor treatment response.
While the cancer is aggressive and recurrence is common, the tumors grow slower in comparison to other types of brain tumors, offering a small window for patient care teams to try different treatment strategies, including the resection of recurrent tumors. Although surgery and radiation can help prolong patient survival, more effective and long-term treatment options are urgently needed, according to Li.
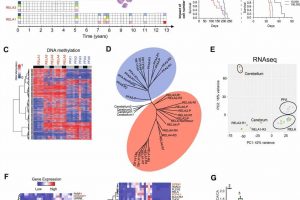
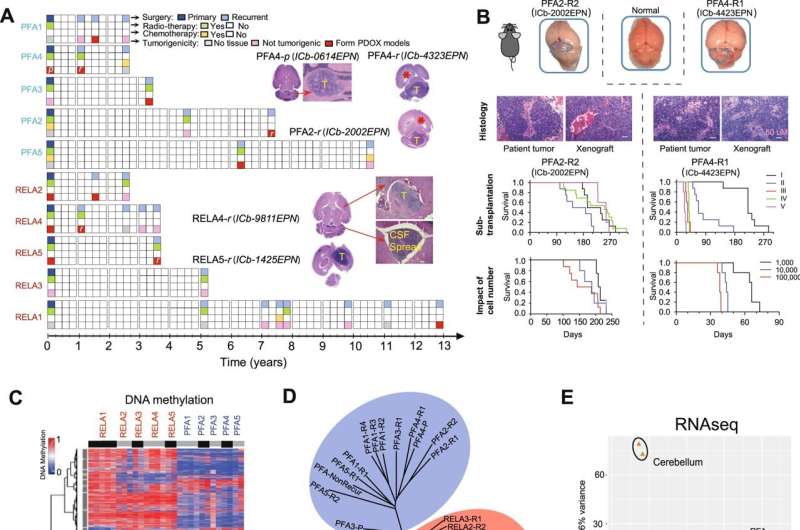
In the current study, Li’s team performed DNA methylation sequencing together with RNA sequencing to identify epigenetic alterations using patient-matched primary and recurring ependymoma tumor tissue samples from 10 pediatric patients who had been treated with radiation and experienced cancer recurrence multiple times over a 13-year period.
From this unique set of patient tumors, the investigators developed a novel panel of patient-derived orthotopic xenograft mouse models, which are critically needed for improving the understanding of tumor biology and for the testing of new therapies, according to Li.
“Ependymoma is a very interesting tumor, and unlike many other human cancers, mutations are not a major cause. In this tumor, we call the mutations ‘silent’ because there’s not a whole lot of mutations. The changes are mostly on the epigenetics side, and that’s why we focus on DNA methylation,” said Li, who is also a professor of Pediatrics in the Division of Hematology, Oncology, and Stem Cell Transplantation.
Using the xenograft models, the investigators identified three unique sets of epigenetic mutations.
The first set included differentially expressed genes that were regulated by potential “driver” DNA methylation regions (DMRs), or genomic regions containing different DNA methylation patterns, that carried over from the primary tumors to recurring tumors.
The second set contained differentially expressed genes that were regulated by potential “booster” DMRs, which were found only in recurring tumors. In particular, the investigators discovered that the gene PLEKHG1 was expressed in all the recurrent tumor models which, according to Li, suggests that this gene could be a potential therapeutic target.
The third set of mutations identified in primary tumors were “predictors of relapse,” or a set of epigenetic markers that could help predict whether a tumor has a high risk of recurrence. These predictors could help providers avoid giving unnecessary treatment to patients which could lead to further complications down the road, according to Li.
“The pediatric brain is still developing, and if you treat them unnecessarily too harsh, it will delay development and decease quality of life. If there’s any way we can predict and improve their quality of life, that’s so important,” Li said.
Furthermore, screening these drivers and boosters could help determine potential therapeutic targets and precision medicine-based treatment strategies.
“The important things to know are that recurrent tumors and not exactly the same as primary tumors—they change a lot—and for this type of study, we need more collaboration. We need to work with as many hospitals and institutions as possible,” Li said.
More information:
Sibo Zhao et al, Epigenetic Alterations of Repeated Relapses in Patient-matched Childhood Ependymomas, Nature Communications (2022). DOI: 10.1038/s41467-022-34514-z
Journal information:
Nature Communications
Source: Read Full Article