Study uncovers mechanism behind primary graft dysfunction
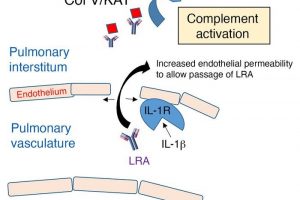
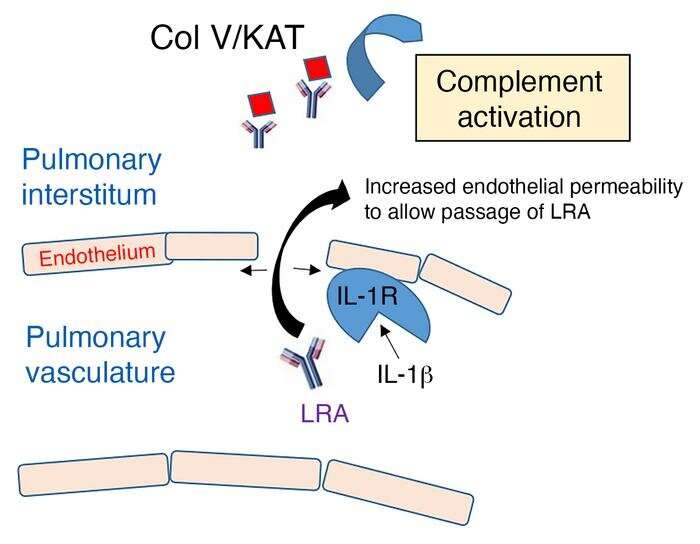
Northwestern Medicine scientists have discovered the pathways through which autoantibodies—immune proteins that mistakenly attack a person’s own body—leak out of blood vessels and cause primary graft dysfunction in some lung transplant recipients, according to findings published in the Journal of Clinical Investigation (JCI).
Primary graft dysfunction (PGD) is a potentially lethal injury to fragile transplanted lungs that occurs in the first days after a transplant operation and affects more than half of lung transplant recipients. The condition is the leading cause of early post-transplantation morbidity and mortality.
In the study, mice were injected with lung-restricted autoantibodies (LRAs) prior to transplantation. Scientists then transplanted a single lung from an untreated mouse and observed the leakage of the LRAs out of vessels and into surrounding tissue—a process called extravasation—which led to rejection of the lung.
Building on previous Northwestern Medicine research which showed that the protein interleukin 1 beta (IL1B) can increase pulmonary vascular permeability and allow cellular extravasation, investigators compared rates of PGD in mice transplanted with donor lungs treated with IL1RA (an IL1 receptor antagonist) or donor lungs from mice genetically modified to modulate IL1-related immune and inflammatory responses. They found IL1B is necessary for the extravasation of LRAs.
In human patients, the investigators analyzed preexisting LRAs in 56 lung transplant recipients and found LRAs could independently predict PGD.
This discovery is already being applied to treat lung transplant patients, said Ankit Bharat, MBBS, the Harold L. and Margaret N. Method Professor of Surgery and chief of Thoracic Surgery and co-senior author of the study.
“We found out the specific pathways that are activated by these autoantibodies which results in a very profound primary graft dysfunction,” Bharat said. “We validated that in human patients, and the important thing is these mechanisms that we have discovered are clinically treatable by specific drugs that are already available and FDA-approved. So, for the first time, we have a specific treatment directed against primary graft dysfunction.”
Historically, Bharat said, treatments for PGD consisted of supportive treatments to buy time in hopes the problem would resolve on its own.
“I would like to emphasize the importance of collaboration between basic scientists like me and physicians who care for patients,” said Emilia Lecuona, Ph.D., research associate professor of Surgery in the Division of Thoracic Surgery and co-senior author of the study. “To be able to apply observations made on patients, ask “Why is this happening?” and take that question and try and solve it in a research bench. I think it’s amazing.”
In the future, Bharat said he aims to develop a test to predict and prevent primary graft dysfunction.
“What we want to do is create a library of all these antigens specific to the different organs and then there can be a high-throughput test where you can take the sample of plasma of the recipient and try to react it to the panel of all the potential antigens that may be present in the lung or any organ to see if they will have primary graft dysfunction and if they do, we can immediately treat them,” said Bharat, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
More information:
Wenbin Yang et al, IL-1β–dependent extravasation of preexisting lung-restricted autoantibodies during lung transplantation activates complement and mediates primary graft dysfunction, Journal of Clinical Investigation (2022). DOI: 10.1172/JCI157975
Journal information:
Journal of Clinical Investigation
Source: Read Full Article