Vaccine printer could help vaccines reach more people
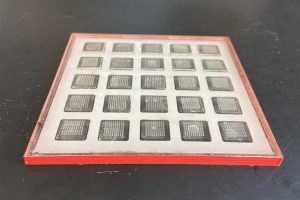
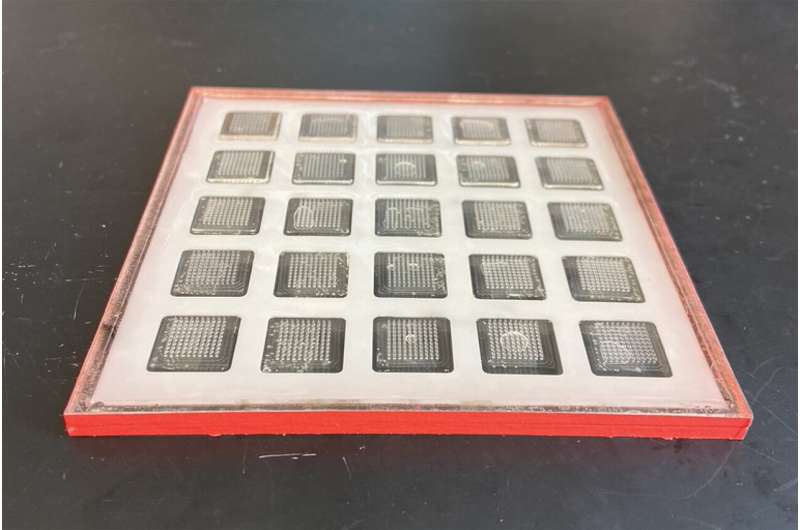
Getting vaccines to people who need them isn’t always easy. Many vaccines require cold storage, making it difficult to ship them to remote areas that don’t have the necessary infrastructure.
MIT researchers have come up with a possible solution to this problem: a mobile vaccine printer that could be scaled up to produce hundreds of vaccine doses in a day. This kind of printer, which can fit on a tabletop, could be deployed anywhere vaccines are needed, the researchers say.
“We could someday have on-demand vaccine production,” says Ana Jaklenec, a research scientist at MIT’s Koch Institute for Integrative Cancer Research. “If, for example, there was an Ebola outbreak in a particular region, one could ship a few of these printers there and vaccinate the people in that location.”
The printer produces patches with hundreds of microneedles containing vaccine. The patch can be attached to the skin, allowing the vaccine to dissolve without the need for a traditional injection. Once printed, the vaccine patches can be stored for months at room temperature.
In a study appearing today (April 24) in Nature Biotechnology, the researchers showed they could use the printer to produce thermostable COVID-19 RNA vaccines that could induce a comparable immune response to that generated by injected RNA vaccines, in mice.
Jaklenec and Robert Langer, the David H. Koch Institute Professor at MIT and a member of the Koch Institute, are the senior authors of the study. The paper’s lead authors are former MIT postdoc Aurelien vander Straeten, former MIT graduate student Morteza Sarmadi ’21, and postdoc John Daristotle.
Printing vaccines
Most vaccines, including mRNA vaccines, have to be refrigerated while stored, making it difficult to stockpile them or send them to locations where those temperatures can’t be maintained. Furthermore, they require syringes, needles, and trained health care professionals to administer them.
To get around this obstacle, the MIT team set out to find a way to produce vaccines on demand. Their original motivation, before COVID-19 arrived, was to build a device that could quickly produce and deploy vaccines during outbreaks of diseases such as Ebola. Such a device could be shipped to a remote village, a refugee camp, or military base to enable rapid vaccination of large numbers of people.
Instead of producing traditional injectable vaccines, the researchers decided to work with a novel type of vaccine delivery based on patches about the size of a thumbnail, which contain hundreds of microneedles. Such vaccines are now in development for many diseases, including polio, measles, and rubella. When the patch is applied to the skin, the tips of the needles dissolve under the skin, releasing the vaccine.
“When COVID-19 started, concerns about vaccine stability and vaccine access motivated us to try to incorporate RNA vaccines into microneedle patches,” Daristotle says.
The “ink” that the researchers use to print the vaccine-containing microneedles includes RNA vaccine molecules that are encapsulated in lipid nanoparticles, which help them to remain stable for long periods of time.
The ink also contains polymers that can be easily molded into the right shape and then remain stable for weeks or months, even when stored at room temperature or higher. The researchers found that a 50/50 combination of polyvinylpyrrolidone and polyvinyl alcohol, both of which are commonly used to form microneedles, had the best combination of stiffness and stability.
Inside the printer, a robotic arm injects ink into microneedle molds, and a vacuum chamber below the mold sucks the ink down to the bottom, making sure that ink reaches all the way to the tips of the needles. Once the molds are filled, they take a day or two to dry. The current prototype can produce 100 patches in 48 hours, but the researchers anticipate that future versions could be designed to have higher capacity.
Antibody response
To test the long-term stability of the vaccines, the researchers first created an ink containing RNA that encodes luciferase, a fluorescent protein. They applied the resulting microneedle patches to mice after being stored at either 4°C or 25°C (room temperature) for up to six months. They also stored one batch of the particles at 37°C for one month.
Under all of these storage conditions, the patches induced a strong fluorescent response when applied to mice. In contrast, the fluorescent response produced by a traditional intramuscular injection of the fluorescent-protein-encoding RNA declined with longer storage times at room temperature.
Then, the researchers tested their COVID-19 microneedle vaccine. They vaccinated mice with two doses of the vaccine, four weeks apart, then measured their antibody response to the virus. Mice vaccinated with the microneedle patch had a similar response to mice vaccinated with a traditional, injected RNA vaccine.
The researchers also saw the same strong antibody response when they vaccinated mice with microneedle patches that had been stored at room temperature for up to three months.
“This work is particularly exciting as it realizes the ability to produce vaccines on demand,” says Joseph DeSimone, a professor of translational medicine and chemical engineering at Stanford University, who was not involved in the research. “With the possibility of scaling up vaccine manufacturing and improved stability at higher temperatures, mobile vaccine printers can facilitate widespread access to RNA vaccines.”
While this study focused on COVID-19 RNA vaccines, the researchers plan to adapt the process to produce other types of vaccines, including vaccines made from proteins or inactivated viruses.
“The ink composition was key in stabilizing mRNA vaccines, but the ink can contain various types of vaccines or even drugs, allowing for flexibility and modularity in what can be delivered using this microneedle platform,” Jaklenec says.
More information:
Robert Langer, A microneedle vaccine printer for thermostable COVID-19 mRNA vaccines, Nature Biotechnology (2023). DOI: 10.1038/s41587-023-01774-z. www.nature.com/articles/s41587-023-01774-z
Journal information:
Nature Biotechnology
Source: Read Full Article