arava operation

The molecular world is full of surprises, and none more stunning than those occurring among the infinitesimal proteins that drive the activities guarding against viral infections and cancer.
In Canada, molecular biologists have found that the activity of a single protein, which strongly influences antiviral and antitumor immune responses, is regulated by the amino acid cysteine.
But what may seem at first blush an arcane discovery involving amino acid chemistry turns out to be a finding of timely significance. A team, led by scientists at Center Hospitalier de l’Université de Montréal, has elucidated in intricate new detail the signaling activity that underlies how emerging cancers are thwarted. And of equal importance—especially in the midst of the global coronavirus pandemic—a finding that sheds new light on a critical piece in the larger jigsaw puzzle of how immune forces are alerted to attack viral infiltrators.
Having unraveled one of nature’s mysteries about a series of key innate immune system activities, information about nexium the scientists have opened a new window of understanding into a biological pathway that can aid the development of new cancer immunotherapies. And the same novel pathway, these scientists say, may hold sway over vaccine development in the not-too-distant future as researchers become ever mindful of the critical first steps in the innate immune response.
The new research centers on what is known as an adapter protein, a molecule formally called STING, which stands for stimulator of interferon genes. STING arrives in an early phase of the innate immune response, and cysteine, an amino acid, regulates STING’s conformation and activity.
But the Canadian team discovered that a specific cysteine residue limits the full antiviral and antitumor effects of STING. The researchers are calling for the development of novel therapeutic interventions that can circumvent the residue, hence allowing the full biological impact of STING.
The Center Hospitalier research also involved molecular biologists at l’Université de Montréal’s departments of biochemistry and molecular medicine who contributed to exploring STING. But while STING may seem an obscure protein, the new research has brought it out of the molecular shadows and into a spotlight. Its role in viral infectious diseases, the scientists say, is pivotal. As soon as a viral infection is detected, STING strikes.
“The adaptor protein STING is stimulated in response to the detection of cytosolic viral DNA by the enzyme cGAS,” wrote Dr. Natalia Zamorano Cuervo and colleagues, referring to the protein, cyclic GMP-AMP synthase, or cGAS. It is one in a cascade of signaling molecules that is in the first wave of innate immune system responses.
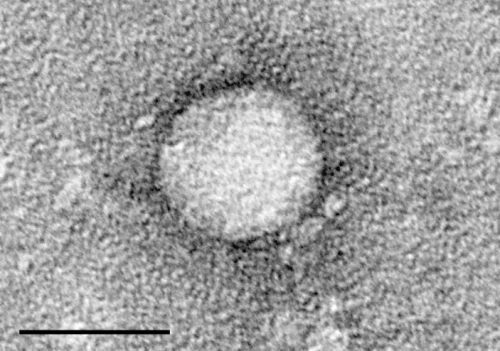
Whenever a virus, such as herpesvirus—a DNA virus—causes infection, its DNA is recognized by cGAS, a sensor of foreign nucleic acids. This, in turn, catalyzes a series of rapid biological events—among them, activating STING. “STING activation then leads to the expression of genes encoding type I interferons as part of the antiviral response. STING activation also plays a role in antitumor immunity, making STING an important therapeutic target, Zamorano Cuervo and her team wrote in the journal Science Signaling.
STING is most vital in the innate immune response to pathogens that take up residence inside cells, namely viruses. Viral DNA in the cellular cytosol activates cGAS, which in turn generates a molecule that activates STING. STING activation triggers genes for type I interferons in the early antiviral response. This basic scenario—cGAS to STING to type I interferons—occurs whether the offender is a virus or a tumor cell. In viral infections, STING’s inducing the production of type I interferons stimulates an immune response that promotes viral clearance.
In addition to DNA viruses, cGAS binds to double-stranded DNA from various other invaders, including bacteria. And while cGAS is a master at binding to foreign DNA, there is evidence that it’s also involved in restricting RNA virus infection. The cGAS-STING research arena is an active area of investigation worldwide. But scientists underscore that many questions about the cGAS-STING signaling axis remain unanswered, part of a still-unexplored frontier.
The Montréal scientists relied on mass spectrometry and structural analyses to identify cysteine residues that underwent reversible oxidation during the activation of STING. One specific residue, oxidized either in response to other oxidants in the cytosolic milieu, or a molecule involved as an activator of STING, led to a loss of STING robustness. Regardless of which of the two is the cause, the team determined that loss as a key limitation. Recognizing that STING activity can become limited, Zamorano Cuervo and collaborators say designing targeted therapeutics can override that limitation and modulate STING’s activity in both viral infections and cancer.
“Pinpointing cysteine oxidation sites by high-resolution proteomics reveals a mechanism of redox-dependent inhibition of human STING,” Zamorano Cuervo and her team concluded.
The loss of robustness, which occurs because of a change in redox status, is not isolated to STING—scientists have seen it before. For example, changes in redox status occur during various cellular processes, including viral infections.
Source: Read Full Article