cheap alli canadian pharmacy no prescription

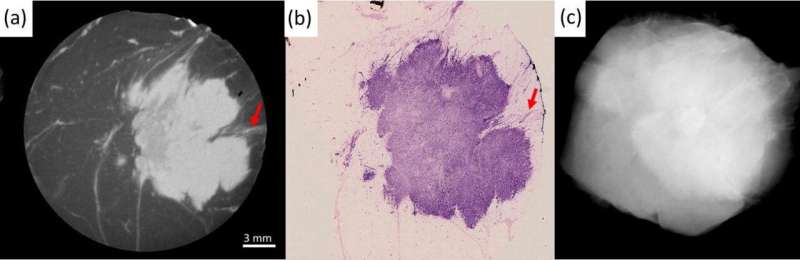 cheap buspar for sale f). he top row focuses on the similarity between the XPCI-CT slice in (b) and the histological slice in (c). Arrow 1 indicates margin involvement, arrow 2 a variation in density in the internal structure of the tumor mass, arrow 3 tumor-induced inflammation. All this is confirmed by the histological slice in (c), and hardly visible in the conventional image in (a). The bottom row focuses on the detection of small calcifications, a key feature in DCIS. These are undetectable in (d), detected in (e), enhanced in the maximum intensity projection (MIP) image at the bottom of (f), and confirmed by histopathology in the top part of (f). The scale bar [shown in (b) and (e)] is the same for all images apart from (f), which has its own scale. Red arrows in (e) and (f) indicate the microcalcifications. Credit: Professor Alessandro Olivo” width=”800″ height=”416″>
cheap buspar for sale f). he top row focuses on the similarity between the XPCI-CT slice in (b) and the histological slice in (c). Arrow 1 indicates margin involvement, arrow 2 a variation in density in the internal structure of the tumor mass, arrow 3 tumor-induced inflammation. All this is confirmed by the histological slice in (c), and hardly visible in the conventional image in (a). The bottom row focuses on the detection of small calcifications, a key feature in DCIS. These are undetectable in (d), detected in (e), enhanced in the maximum intensity projection (MIP) image at the bottom of (f), and confirmed by histopathology in the top part of (f). The scale bar [shown in (b) and (e)] is the same for all images apart from (f), which has its own scale. Red arrows in (e) and (f) indicate the microcalcifications. Credit: Professor Alessandro Olivo” width=”800″ height=”416″>
A new X-ray imaging scanner to help surgeons performing breast tumor removal surgery has been developed by UCL experts.
Most breast cancer operations are what are known as conserving surgeries, which remove the cancerous tumor rather than the whole breast. Second operations are often required if the margins (edges) of the extracted tissue are found to not be clear of cancer.
Researchers at UCL, Queen Mary University of London, Barts Health NHS Trust and Nikon used a new approach to X-ray imaging which allows surgeons to assess extracted tissue intraoperatively, or during the initial surgery, giving 2.5 times better detection of diseased tissue in the margins than with standard imaging.
In the paper, published today in Scientific Reports, academics use X-Ray Phase Contrast Imaging (XPCI), developing a scanner which provides surgeons with a full 3-D image of the extracted tissue lump, known as a wide local excision (WLE).
Currently, the WLE is assessed through histopathology—the microscopic examination of tissue—with results only available after several days. If affected margins are detected, often a second operation is required.
Lead author Professor Alessandro Olivo (UCL Medical Physics & Biomedical Engineering) said: “I am terribly excited about these results, as they are likely to lead to the first clinical use of XPCI. The technology has tremendous potential, and I am sure once people see what it can do many other clinical areas will follow suit.”
So far, most proposed approaches to allowing an intraoperative assessment had too many shortcomings to be effective, either in the ability to detect all of the diseased tissue or in achieving a sufficient penetration depth in the specimen, or in both.
Researchers tested the scanner on 101 WLEs and compared the results with the current standard method used intraoperatively (based on conventional X-rays). The superior detection rate of 2.5 times could result in a similar reduction in the re-operation rate.
Co-author Professor Louise Jones (Director, Breast Cancer Now Tissue Bank) said: “This technology has the potential to significantly improve radiological intraoperative assessment of tumor margins, potentially reducing the need for repeat operations that many patients require, which can cause significant distress to the patient.”
Co-author Tamara Suaris (Consultant Breast Radiologist, Barts NHS Health Trust) added: “Although we have focused on the impact of XPCI in breast surgery, there is a much wider clinical potential of this technology including other intraoperative areas such as intestinal, oesophageal and prostatic surgery and in the longer term, diagnostic imaging, notably mammography.”
XPCI imaging provides soft tissue sensitivity which is superior to conventional X-ray. Whereas standard imaging picks up the X-ray beam’s change in intensity as it travels through tissue, phase contrast imaging measures the changes in speed with which X-ray travels through different tissues, which has been proven to enhance soft tissue contrast, including of breast tumors.
Source: Read Full Article