crestor equal lipitor
In many ways, our intestines serve as the front gate between our bodies and the world around us. They help digest our food and absorb our medications. They send alarm signals when harmful bacteria, viruses, and allergens invade.
When the intestines malfunction, or fail to form properly during development, a wide range of gastrointestinal diseases can occur; such as inflammatory bowel disease (IBD), eosinophilic esophagitis (EoE), and celiac disease. Many infectious diseases, ranging from mild to deadly, also start in the intestine.
Now, pantoprazole 40 mg gastritis scientists at Cincinnati Children’s report a significant breakthrough in a years-long quest to build a better model for studying gastrointestinal disease. They have succeeded at developing a complex, next-generation intestinal organoid that includes key elements of a functional immune system.
Detailed findings were published online Jan. 26, 2023, in Nature Biotechnology. The co-authors say this is the first in vivo organoid of any type (heart, liver, stomach, etc.) to incorporate a functional immune system.
“Not only does the organoid support the migrating immune tissues—rather than rejecting them—but those immune cells and structures go on to improve the development of the intestine itself, specifically its ability to recognize foreign antigens,” says corresponding author Michael Helmrath, MD.
Five-year project blends ‘humanized’ mouse and organoid tech
Many of the experiments that helped confirm the new findings were led by first author Carine Bouffi, Ph.D., then a member of Helmrath’s lab at Cincinnati Children’s and now a senior scientist at the pharmaceutical company Boehringer Ingelheim. Overall, the work involved 20 scientists at five institutions.
The work started with growing intestine organoids following an innovative process created in 2011 by experts with the Center for Stem Cell & Organoid Medicine (CuSTOM) at Cincinnati Children’s. That team was the first in the world to produce a functioning intestine organoid from induced pluripotent stem cells (iPSCs), a type of stem cell made from mature human cells instead of fetal tissue. The center has achieved several milestones since, including producing the world’s first esophagus and stomach organoids.
After growing for 28 days in a dish, the tiny organoid spheres had reached a few millimeters in size. Then came the next crucial step: transplanting the organoids into an immunosuppressed mouse to avoid rejection of the human tissue.
In this study, to investigate the role of the immune system during development, the team used “humanized” mice that had been genetically modified to suppress their own immune systems, which allowed transplanted human immune cells to function instead. Many research centers around the world use similar humanized mice in their projects.
The team members performed delicate surgery to transplant the organoids into mice by placing them under a membrane surrounding their kidneys. There, the organoids continued to grow more than 1,000-fold to about 1 cm in diameter (about the size of a pea).
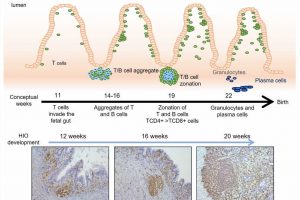
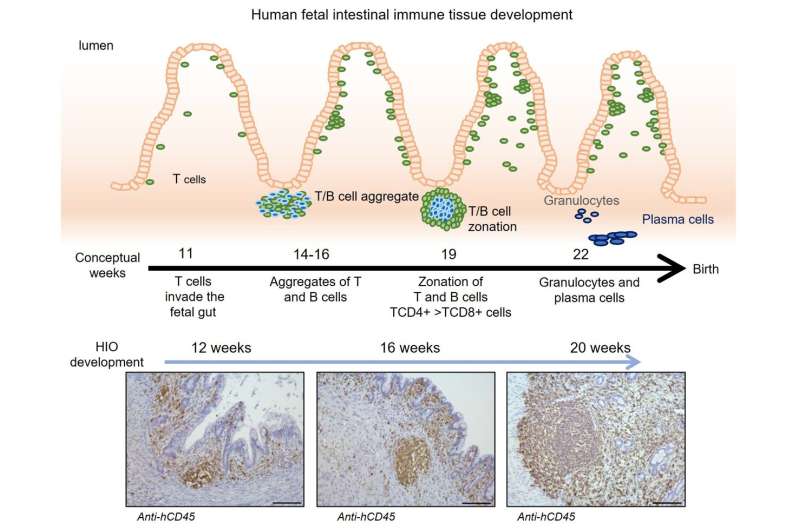
At 12, 16 and 20 weeks, the lab team collected organoids to analyze the types of cells they contained. They found that the organoids contained multiple types of human immune cells that migrated from the humanized mice, including CD45+ cells and important cell formations called gut-associated lymphoid tissue (GALT) structures.
“We saw that immune cells from the humanized mouse migrated to the organoid,” Helmrath says. “We saw the same types of cells, following the same sequences of development, that we see in human development.”
Importantly, the team further confirmed that functional M cells—a key type of immune signaling cell—were produced only when humanized tissues were exposed to specific bacterial antigens. Achieving this required exposing the inside of the tiny developing intestines to fragments of E. coli bacteria, a microbe normally found in human intestine. This triggered the organoid tissue to secrete antibodies in reaction to the exposure.
“When we started this project, we did not know if implanting intestinal organoids in humanized mice would work, since it had never been done before,” Bouffi says. “When we got the first results, we saw the infiltration of immune cells in the organoids and I thought that the host mouse was rejecting the organoid. But after further analysis, we found that the immune cells were actually organized and later developed into an immune tissue comparable to a human intestine. Organoids are remarkable.”
Potential uses for next-gen intestinal organoids
The first applications for these improved organoids will likely involve using them as testing platforms to learn more about the many immune-mediated diseases affecting the digestive tract, Helmrath says. Unlike animal models, organoids can be grown to more accurately reflect the genetic conditions of a common disease state or even to act as “avatars” that mimic organ tissue from a specific patient.
“There are broad potential applications to these findings, not just for intestine organoids but for all organs affected by immune-mediated disease,” Helmrath says.
Longer term, scientists hope to replicate their success in mice with larger animal hosts that can produce larger volumes of human organoid tissue. If successful, lab-started organ tissues eventually might serve as living patches that could help damaged organs heal themselves before people require full-organ transplantation.
More information:
Carine Bouffi et al, In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice, Nature Biotechnology (2023). DOI: 10.1038/s41587-022-01558-x
Journal information:
Nature Biotechnology
Source: Read Full Article