furosemide electrolyte imbalance
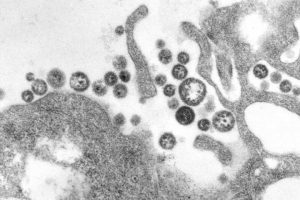
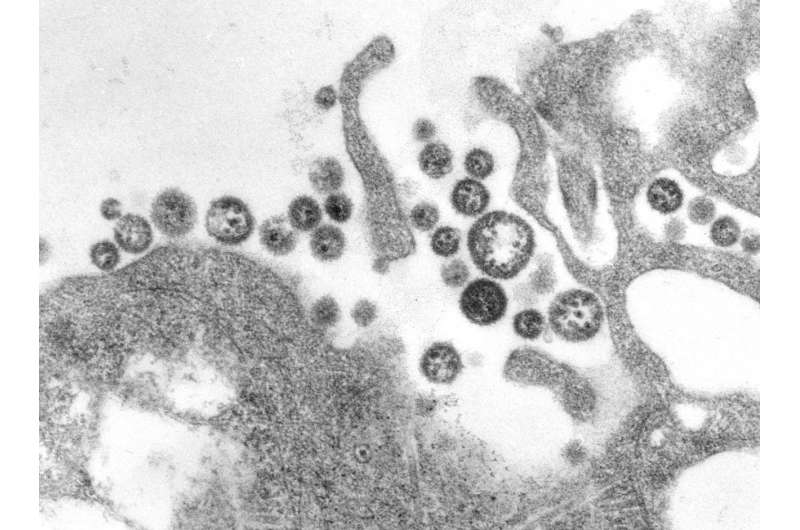
Lassa fever is a viral illness that is far too common in West Africa. Although it can have a mortality rate of 15% in severe cases, up to 90% in pregnant women, and causes deafness in a quarter of survivors, there is no vaccine or antiviral to protect against Lassa virus. To save lives, scientists at La Jolla Institute for Immunology (LJI) and Scripps Research are working to understand exactly how Lassa virus replicates within human hosts.
In a new study, published in Proceedings of the National Academy of Sciences, the researchers show how a critical Lassa virus protein, called polymerase, hep c and folic acid drives infection by harnessing a cellular protein in human hosts. Their work suggests future therapies could target this interaction to treat patients.
“There is no antiviral drug that specifically targets Lassa virus,” explains study first author Jingru Fang, a joint LJI and Scripps Research graduate student. “That’s why it’s important for researchers to identify potential druggable targets on this virus to combat infection.”
Lassa virus encodes only four viral proteins. One of them, the polymerase, directs the process of virus genome replication and gene expression to produce the materials the virus needs to spread to new host cells. If one can stop virus polymerase, one can stop infection.
Together with study senior authors LJI President and CEO Erica Ollmann Saphire, Ph.D., and Scripps Research Professor Juan De La Torre, Ph.D., Fang led the hunt for host cellular proteins that may act as Lassa polymerase’s partners in crime.
The hunt for Lassa’s helper
Fang and her colleagues engineered Lassa virus polymerase to carry an enzymatic tag that labels polymerase-interacting host proteins with a special chemical handle. The researchers then fished out host proteins with this chemical handle and used a technique called mass spectrometry to identify these host proteins that interact with Lassa virus polymerase.
“It is like defining the Lassa virus polymerase social network, which allows you to go after partners,” says De La Torre.
In collaboration with Professor Alexander Bukreyev, Ph.D., and colleagues at University of Texas Medical Branch (UTMB), the team carried out a “function screen” using live Lassa virus. This work, carried out inside a high-containment laboratory, revealed which of these host proteins could be important for Lassa infection. Among a total of 42 host proteins that interact with Lassa polymerase, the team focused on one druggable target: GSPT1. The team showed that GSPT1 is physically and functionally linked to Lassa virus polymerase and can facilitate Lassa virus infection.
This study is the first to uncover molecular cross-talks between Lassa virus polymerase and cellular proteins. However, it is the second-ever time the host protein GSPT1 has been linked to virus infection. The first was a recent Cell Reports study showing viral polymerase hijacking GSPT1 in Ebola virus infections—research also led by Saphire, De La Torre, and Fang.
“If we could find a way to either disrupt the link between GSPT1 and Lassa polymerase, or if we could simply remove GSPT1 protein, we could stop Lassa virus infection,” says Fang.
Eyeing a new drug for Lassa
To their surprise, the team found a drug candidate, called CC-90009, which has been shown to destroy GSPT1 proteins and is currently being examined as a cancer therapy in clinical trials.
To see whether they can repurpose the existing GSPT1 inhibitor against Lassa infection, Research Associate Colette Pietzsch, from Bukreyev group at UTMB, added CC-90009 to Lassa-infected human liver cells inside a high-containment laboratory. This experiment showed that CC-90009 treatment significantly reduced Lassa virus growth without obvious cell toxicity.
The researchers say it is feasible that this same small molecule drug could double as an Ebola virus therapy, and their Cell Reports data suggest that CC-90009 can reduce virus titer at later time points of Ebola virus infection.
Source: Read Full Article