metrogel medicine
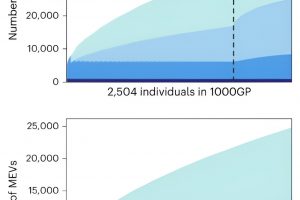
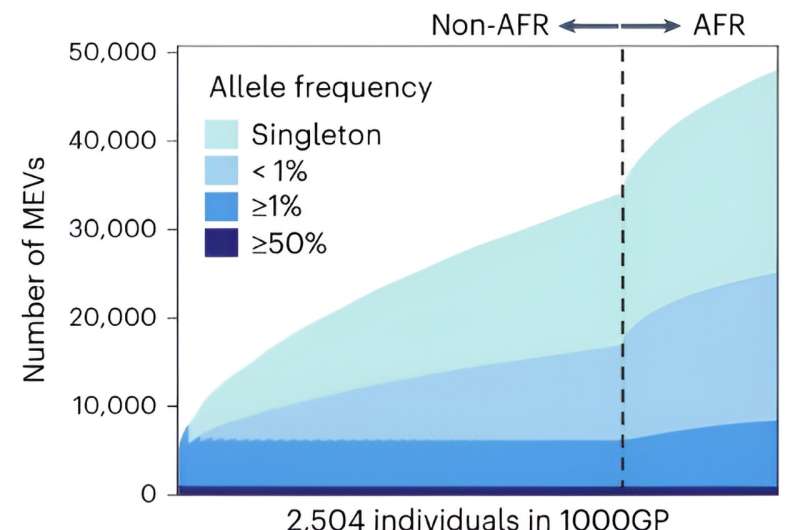
RIKEN geneticists have developed a tool that can quickly and accurately analyze variants in mobile genetic elements, commonly known as “jumping genes.” This promises to shed light on the role such variants play in disease.
Most genes stay put within the genome, but a few have the ability to jump around and insert themselves in different locations. Known as mobile elements, these snippets of DNA move around by employing a “copy and paste” mechanism.
Mobile element variants (MEVs) speed up the genomic divergence of populations of a species. They can also give rise to diseases.
“Just under half of the human genome is estimated to have originated from mobile elements,” explains Shohei Kojima of the RIKEN Center for Integrative Medical Sciences (IMS). “But the vast majority of genes have lost their mobility due to the accumulation of mutations; only a small proportion can mobilize and have the potential to cause diseases.”
Despite their importance, ibuprofen dosage calculator by weight mobile elements have not been well studied, mainly due to the low accuracy of methods used to analyze them.
“There was no good algorithm for genotyping MEVs, which was a hurdle for studying them at population scale,” says Kojima. “MEVs were overlooked in statistical genetics—which aims to detect the genetic basis of disease using population-scale genetic data—because it requires precise genotyping.”
Now, a team led by Nicholas Parrish, also of IMS, and Kojima has devised a rapid technique that can accurately genotype MEVs. The study, “Mobile element variation contributes to population-specific genome diversification, gene regulation and disease risk,” is published in the journal Nature Genetics.
At the heart of their method is an algorithm that fits a statistical model to short-read, whole-genome sequencing data. To realize a high accuracy, the algorithm draws on multiple sources of evidence.
To demonstrate the potential of their technique, the team applied it to genetic data from about 3,000 non-Japanese people and about 5,000 Japanese people.
This comparison revealed key differences between populations. “We found that East Asians, including Japanese, have more LINE-1 and SVA variations than South Asians and Europeans,” says Kojima. “This was a complete surprise to us. It may explain some East Asian-specific disease risk.”
The team pinpointed a specific MEV that is a risk factor for keloid, a disease characterized by abnormal scar formation. This could have applications for personalized medicine. For example, patients could be tested for this MEV before surgery to estimate the risk of keloid formation.
“Our results show that MEVs are involved in disease risk and gene regulation and contribute to differences between human populations,” says Kojima. “MEVs are a double-edged sword—they can drive human evolution, but occasionally they can cause disease. Our study backs this concept up using population-scale statistical genetics.”
The team intends to apply their tool to rare MEVs and MEVs in somatic cells.
More information:
Shohei Kojima et al, Mobile element variation contributes to population-specific genome diversification, gene regulation and disease risk, Nature Genetics (2023). DOI: 10.1038/s41588-023-01390-2
Journal information:
Nature Genetics
Source: Read Full Article