FDA OKs Motixafortide for Stem Cell Mobilization in Myeloma
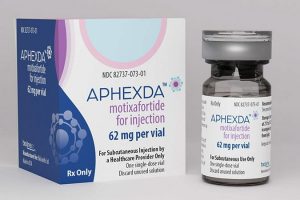
The US Food and Drug Administration (FDA) has approved motixafortide (Aphexda, BioLineRx) in combination with filgrastim (G-CSF) to mobilize hematopoietic stem cells for collection and subsequent autologous transplantation in patients with multiple myeloma. The success of autologous stem cell transplantation (ASCT) depends on adequate mobilization of stem cells during the treatment process. Collection of a sufficient number of stem cells […]
» Read more