abilify coupon for copay

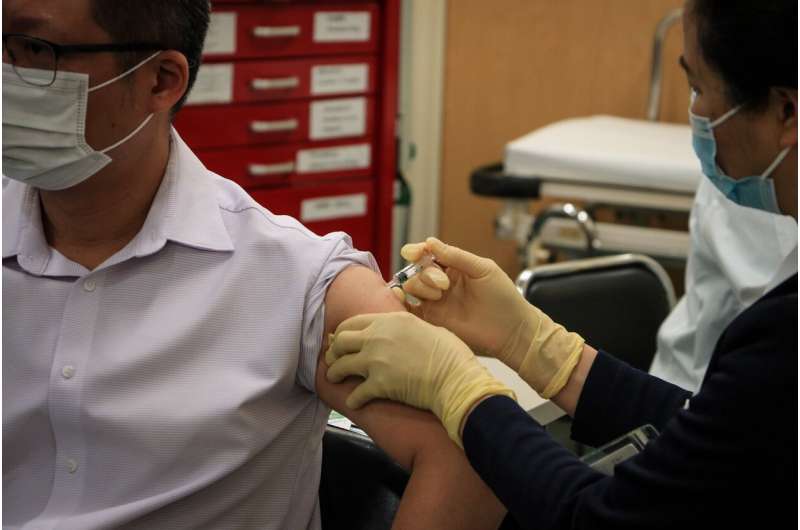
Spain’s medicines agency approved Tuesday a first round of clinical trials on humans for a COVID-19 vaccine developed by Spanish firm Hipra.
“This is the first trial on humans of a vaccine made in Spain,” the agency said in a statement.
Dozens of volunteers will be recruited from Spanish hospitals “as soon as possible” for the clinical trials, it added.
Hipra, which is based in the northeastern city of Girona, said it could produce up to 400 million doses of the vaccine in 2022 and 1.2 billion in 2023 if the jab is approved.
“Today we have taken a big step in the fight against the pandemic,” Prime Minister Pedro Sanchez said in tweet shortly after the agency approved the trials.
Hipra, which has manufacturing bases in Spain and Brazil, has been working on two COVID-19 vaccines.
One is based on the same RNA messenger technology used in Pfizer and Moderna’s shots, amoxicillin and amoxicillin trihydrate while the second, which has just received approval for trial, uses a recombinant protein like US-based drugmaker Novavax.
The European Union is currently using four COVID-19 vaccines: the one from Pfizer, which forms the backbone of its vaccination rollout, as well as those from AstraZeneca, Moderna and Johnson and Johnson.
The Pfizer vaccine must be stored at minus 70 degrees Celsius (minus 94 degrees Fahrenheit), making it a challenge to ship and protect.
Source: Read Full Article