Clinical trial suggests Parkinson’s drug is safe in humans
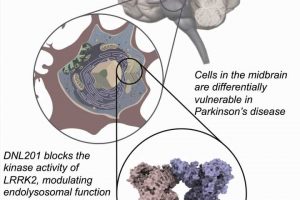
A large team of researchers from Denali Therapeutics, working with colleagues from multiple entities in the U.S. and one in Canada, has found that a LRRK2 inhibitor called DNL201 showed no ill effects to volunteers in a clinical trial. In their paper published in Science Translational Medicine, the group describes their clinical trial of the Parkinson’s drug and what they learned during its run. Patrick Lewis, with Royal Veterinary College London has published a Focus piece in the same journal issue outlining the work being done by the team at Denali.
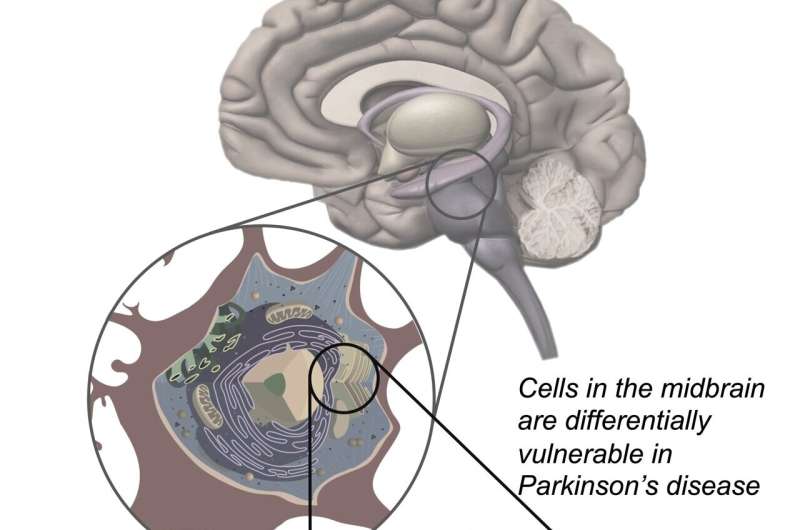
Parkinson’s disease is a disease that results from the destruction of neurons in the brain that produce dopamine, which is critical for motor function. Prior research has suggested it comes about most often due to environmental factors in people with a genetic risk for it. Prior research has also shown that mutated versions of a certain gene lead to overproduction of an enzyme called LRRK2, which leads to inflammation and other problems. Currently, there are no therapies available to slow its progression.
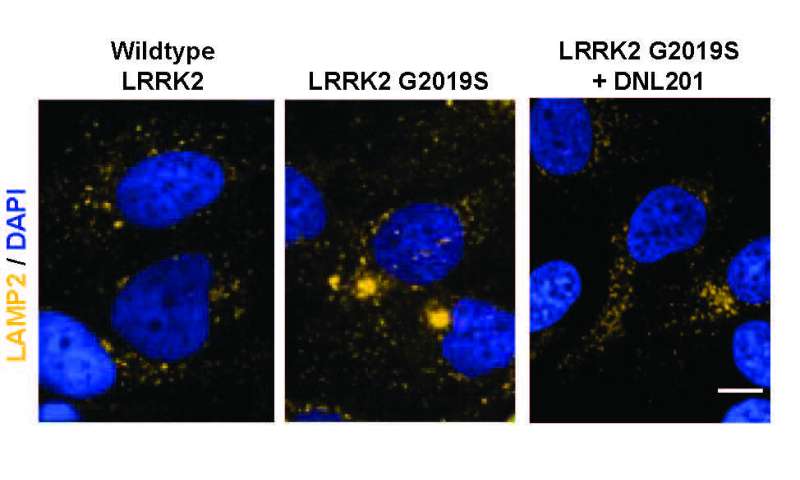
One of the hallmarks of Parkinson’s is elevated levels of the LRRK2 enzyme which damage lysosomes—organelles responsible for removing toxins from cells in the brain. This leads to a buildup of toxins and cell death. Prior work involved in developing drugs to stop the progression of Parkinson’s has centered around reducing such levels. In this new effort, the researchers have developed a drug called DNL201 that has shown promise. It has been tested in animals, where it has effectively reduced LRRK2 enzyme levels. But in some animals, it has also led to a dangerous side effect, where sacks inside cells in the lungs and kidneys swell. In this new clinical trial, the goal was to determine if DNL201 was safe for use in humans, not to assess whether it slowed the progression of Parkinson’s.
Source: Read Full Article