A comparison of SARS-CoV-2 antibody profiles in elderly and younger individuals

In a recent Scientific Reports study, researchers comparatively evaluate antibody titers after double vaccination with the Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) vaccine against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among very elderly individuals over the age of 90 and a younger population between the ages of 23 and 69 years.
Previous studies have reported a progressive decrease in responses and remodeling of the immune system with advancing age, thus causing the effectiveness of SARS-CoV-2 vaccines to vary with age. In particular, elderly patients are at an increased risk of severe coronavirus disease 2019 (COVID-19); however, serological data on elderly individuals over 90 years of age after COVID-19 vaccination is limited.

Study: Six months SARS-CoV-2 serology in a cohort of mRNA vaccinated subjects over 90 years old. Image Credit: Stock-Asso / Shutterstock.com
About the study
In the present cross-sectional study, researchers assess anti-SARS-CoV-2 humoral immune responses in the very elderly and compared their findings to data acquired from a younger population between the ages of 23 and 69 years after BNT162b2 vaccination in Italy.
Data were obtained from post-vaccination serological reports of anti-SARS-CoV-2 antibodies in the very elderly and younger/general population. These samples were collected as part of the COVIDIAG-NOSTIX project between December 2020 and September 2021.
The younger population consisted of healthcare workers who were recruited in a COVID-19 vaccination campaign in Italy. Comparatively, the very elderly population consisted of individuals who received home care assistance from the Operatori Sanitari Association (OSA) Cooperative for medication and rehabilitation purposes.
Sera were obtained from the study participants after six months of double mRNA BNT162b2 vaccination. The elderly cohort additionally completed rapid antibody testing during serum sample collection.
Antibodies against the SARS-CoV-2 spike (S) protein receptor binding domain (RBD) and the nucleocapsid (N) protein were evaluated using electrochemiluminescence immunoassays (ECLIA). The rapid antibody tests involved chemiluminescence (CLIA) assays for detecting anti-SARS-CoV-2 S immunoglobulin G (IgG).
Chromatographic assay-based rapid antibody tests were also performed to detect anti-S and anti-N IgM and IgG titers. These tests were completed in 10 minutes and allowed the researchers to obtain immediate results for the very elderly.
Study findings
The very elderly and younger population included 97 and 1,114 individuals, respectively. Follow-up periods and the proportions of individuals with anti-SARS-CoV-2 N seropositivity of 8-9% and women were similar across the study participants.
Significant differences were observed in anti-SARS-CoV-2 S titers among anti-N seropositive and seronegative participants, with median values of 2,500 U/mL and 587 U/mL in the younger population, respectively. The corresponding values of 2,500 U/mL and 65 U/mL were reported in the very elderly population, respectively.
Considering only seronegative individuals, a significant decrease was noted among both groups, with a lower humoral response observed in the very elderly. Sex-based immune differences were only noted in the younger population.
Over 60% of seropositive cases among the younger group were women. Comparatively, none of the male participants exhibited seropositivity in the very elderly group, which only included seven women. Therefore, comparisons could be made among females only. To this end, no significant differences among the younger and very elderly participants were observed.
Anti-N seropositivity and age were the most important factors in determining anti-SARS-CoV-2 S antibody levels, followed by sex, which produced significant but limited effects on the overall results. Among the very elderly participants, identical differences were observed between anti-SARS-CoV-2 N seropositive and seronegative individuals, with median values of 2,080 U/mL and 42.5 UA/mL reported among anti-N seropositive and seronegative individuals, respectively.
The rapid antibody tests were capable of detecting anti-S IgG antibodies, with positive serum antibody titers exceeding 197 UA/mL in the Roche Elecsys test with specificity and sensitivity of 83% and 100%, respectively. Additionally, correlations were noted between good serological test reports and positive immune levels on rapid antibody tests with median values of 3,263 UA/mL, 899 UA/mL, and 809 UA/mL for strongly positive, positive, and slightly positive reports, respectively.
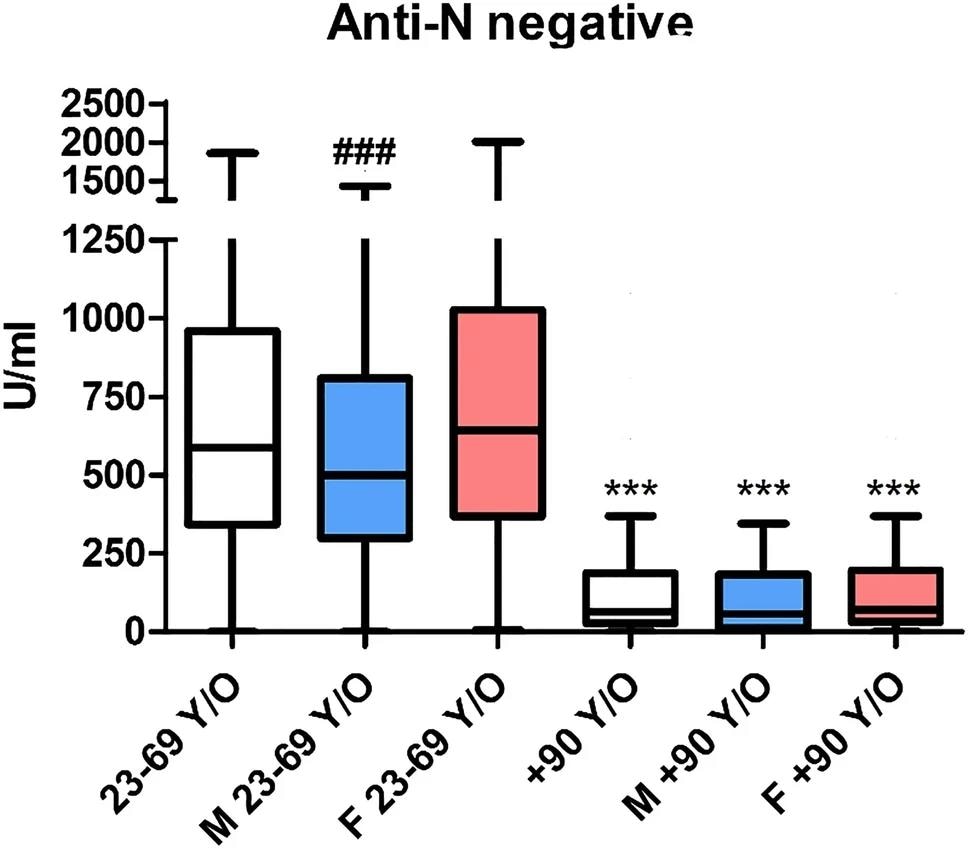
Anti-S antibody titer (by the Roche Elecsys SARS-CoV-2-S) in individuals seronegative for anti-N. The blue box (M) indicates male subjects, and the red one (F) indicates female subjects. ***p < 0.001 versus the correspondent color in the 23–69 y/o cohort; ### p < 0.001 versus females in the same cohort.
Conclusions
The study findings demonstrate that very elderly individuals elicited detectable anti-SARS-CoV-2 S titers after the second BNT162b2 vaccination. This response was primarily observed among anti-N seropositive individuals who had a history of prior COVID-19. In this group, anti-N titers were comparable with that of the younger cohort.
Likewise, the anti-N seronegative elderly exhibited fewer anti-S antibodies as compared to the younger cohort. Therefore, the SARS-CoV-2-specific antibody responses varied on the basis of the anti-N serostatus, individual’s age, and sex among the very elderly.
The present study was the first of its kind to provide data post-vaccination among individuals over the age of 90. These findings could potentially impact COVID-19 vaccination campaign organization, including booster dose administration and prioritization of vaccine recipients.
Further, anti-SARS-CoV-2 antibody titers could be used as support indices for establishing the need for additional vaccine doses, especially among the elderly population, whose immune responses to infections may not be as efficient as those of immunocompetent individuals with a history of COVID-19.
- Tomaiuolo, R., Di Resta, C., Viganò, M. et al. (2022). Six months SARS-CoV-2 serology in a cohort of mRNA vaccinated subjects over 90 years old. Scientific Reports 12(12446). doi:10.1038/s41598-022-15148-z.
Posted in: Men's Health News | Medical Research News | Medical Condition News | Women's Health News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Healthcare, Home Care, Immune System, Immunoassays, Immunoglobulin, Protein, Receptor, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Serological Test, Serology, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Pooja Toshniwal Paharia
Dr. based clinical-radiological diagnosis and management of oral lesions and conditions and associated maxillofacial disorders.
Source: Read Full Article