Humoral responses to SARS-CoV-2 are detectable through saliva, scientists find

Your saliva may be able to tell you if you’ve developed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies, suggests a new medRxiv* preprint study. In immunocompromised groups, salivary Immunoglobulin G (IgG) antibody levels were associated with IgG titers in blood serum 3 to 4 weeks after mRNA vaccination.
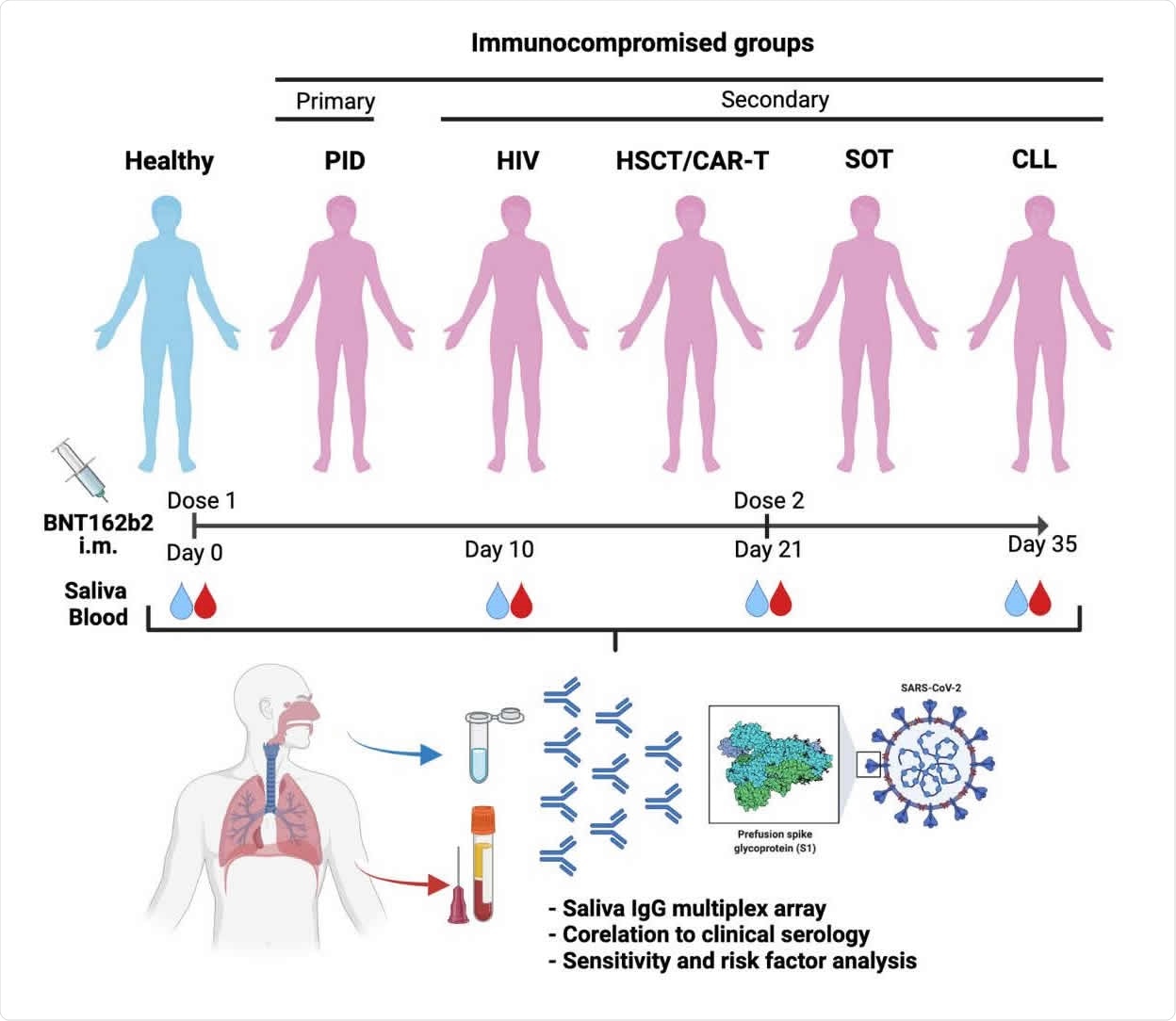
Immunocompromised patients develop lower antibody responses compared to healthy individuals. As a high-risk group for developing severe COVID-19 infection, they require regular monitoring.
“The magnitude of anti-spike IgG responses in the saliva of vaccinated individuals, which exceeded those seen in mild convalescent individuals, is encouraging as it indicates that vaccination might confer a sterilizing immune response in the oral cavity and thereby lower virus transmission,” conclude the researchers.
Based on the study’s results, taking saliva samples may provide a safe and convenient way to assess immunity in this vulnerable population.
How they did it
About 404 immunocompromised patients and 82 healthy participants enrolled in the study. At the time of the research study, all participants were confirmed to have no antibodies for SARS-CoV-2 in their blood and did not test positive for SARS-CoV-2 infection.
All patients received two doses of the Pfizer-BioNTech vaccine. Both saliva and serum samples were collected before the vaccination, 10 days after, 21 days after, and 35 days after the first dose. The second vaccine dose was given 3 weeks after the first dose.
IgG levels were measured because prior research suggested that the Pfizer-BioNTech vaccine elicits more IgG levels than IgA levels in saliva.
A total of 1,870 saliva samples and 1,829 serum samples were collected throughout the study. Saliva flow was normal for a majority of participants. However, patients with CLL or primary immunodeficiencies had a lower saliva flow rate.
Differences in IgG responses in saliva
All patients showed signs of IgG antibodies in their saliva samples after the first vaccine dose.
People with HIV had a 12-fold, and healthy patients had a 12-fold increase of IgG reactivity in saliva. After the second dose, people with HIV exhibited a 53-fold, and healthy patients showed a 74-fold increase.
More than 90% of people with HIV and healthy patients showed an IgG response in salivary samples 35 days after the first dose.
In patients with allogeneic hematopoietic stem cell transplantation (HSCT)/chimeric antigen receptor T (CAR-T) cell therapy, there was a moderate 3-fold increase in IgG reactivity in saliva 21 days after vaccination. However, after the second dose, IgG reactivity rose from 3-fold to 50-fold, suggesting a robust immune response.
In people with chronic lymphocytic leukemia (CLL), organ transplants, and primary immunodeficiencies, weak humoral responses were observed. These groups did not experience detectable IgG levels in saliva until two weeks after the second vaccination. They also had lower anti-Spike-f and anti-S1 responses in saliva than people with HIV or HSCT/CAR-T. The researchers suggest the poor response after vaccination may be due to disease or immunosuppressants.
IgG response in the saliva is associated with IgG antibody titers in blood serum
IgG reactivity in saliva samples was moderately associated with IgG levels 10 days after the first vaccine dose. However, the correlation between IgG levels in saliva and serum grew stronger after receiving the second dose and 2 weeks after the second dose.
In patients with CLL, HSCT/CAR-T, and primary immunodeficiency, having Spike-f or S1 IgG reactivity in salivary samples was strongly associated with serum anti-S1 antibody titers 2 weeks after the second dose.
People living with HIV showed a moderate association between IgG levels in saliva and serum after full vaccination.
Being younger than 60 years correlated with having more potent serum SARS-CoV-2 specific IgG antibody titers.
Study limitations
The researchers did not incorporate an antibody isotype analysis and their neutralizing potency against SARS-CoV-2. Several SARS-CoV-2 variants, such as Delta, contain spike protein mutations that allow it to evade or weaken neutralizing antibodies.
Immunity against SARS-CoV-2 is not limited to neutralizing antibodies. Local memory B and T cell immunity play an essential role against SARS-CoV-2. However, the presence of B and T cells in saliva samples was not explored in the current study.
Additionally, patients enrolled in the study were assessed for humoral immunity after receiving the Pfizer-BioNTech vaccine, but how this fares up with other coronavirus vaccines remain unknown.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Healy K, et al. (2021). Appearance of IgG to SARS-CoV-2 in saliva effectively indicates seroconversion in mRNA vaccinated immunocompromised individuals. medRxiv. Doi: https://doi.org/10.1101/2021.09.30.21264377, https://www.medrxiv.org/content/10.1101/2021.09.30.21264377v1
Posted in: Medical Research News | Disease/Infection News
Tags: Antibodies, Antibody, Antigen, Blood, Cell, Chimeric Antigen Receptor, Chronic, Chronic Lymphocytic Leukemia, Coronavirus, Coronavirus Disease COVID-19, HIV, Immune Response, immunity, Immunodeficiency, Immunoglobulin, Leukemia, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Jocelyn Solis-Moreira
Jocelyn Solis-Moreira graduated with a Bachelor's in Integrative Neuroscience, where she then pursued graduate research looking at the long-term effects of adolescent binge drinking on the brain's neurochemistry in adulthood.
Source: Read Full Article