SARS-CoV-2 viable 21 days in blood, mucus, semen, urine, but just 24 hours in breast milk

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes the coronavirus disease (COVID-19), spreads through respiratory droplets and aerosols expelled when infected individuals cough, sneeze, breathe or sing.
Previous studies have demonstrated SARS-CoV-2’s stability on various surface types and seasonal climatic conditions. SARS-CoV-2 can survive the longest on surfaces in winter, followed by spring and fall.
Researchers at Kansas State University showed that SARS-CoV-2 is stable for 21 days in nasal mucus, saliva, sputum, tear, saliva, blood, urine, and semen. It also remained infectious longer during winter, spring, and fall conditions than in summer conditions.
Meanwhile, SARS-CoV-2 was only stable for about 24 hours in breast milk and feces. The study findings, which appeared in the pre-print server bioRxiv*, highlight the potential risk of infectious body fluids in SARS-CoV-2 transmission.
SARS-CoV-2 transmission
Since the coronavirus disease first emerged in China, it has spread globally and impacted economies. It has posed a considerable threat to global public health, infecting more than 136 million people and claiming the lives of more than 2.94 million globally.
SARS-CoV-2’s transmission is primarily mediated through close contact with an infected person who sheds respiratory droplets. Droplets come in many sizes, wherein respiratory droplets are more than 5 μm in diameter. Meanwhile, smaller droplets that are less than 5 μm in diameter spread via airborne transmission. These droplets evaporate quickly to produce infectious aerosols that can remain suspended in the air for many hours and travel over a long distance.
Another route of transmission includes exposure to the infectious virus on surfaces. Droplets tend to settle down to the ground or on surfaces within a limited distance.
The virus can survive for several days, depending on the type of surface. Also, previous studies have shown that infectious droplets thrive longer on surfaces during the cold season than during the summer.
Scientists investigated the presence of SARS-CoV-2 in biological fluids since it serves as a possible infection source. Overall, nasal mucus, sputum, and saliva are significant components to generate the respiratory droplets where transmission of SARS-CoV-2 mainly occurs.
SARS-CoV-2 stability
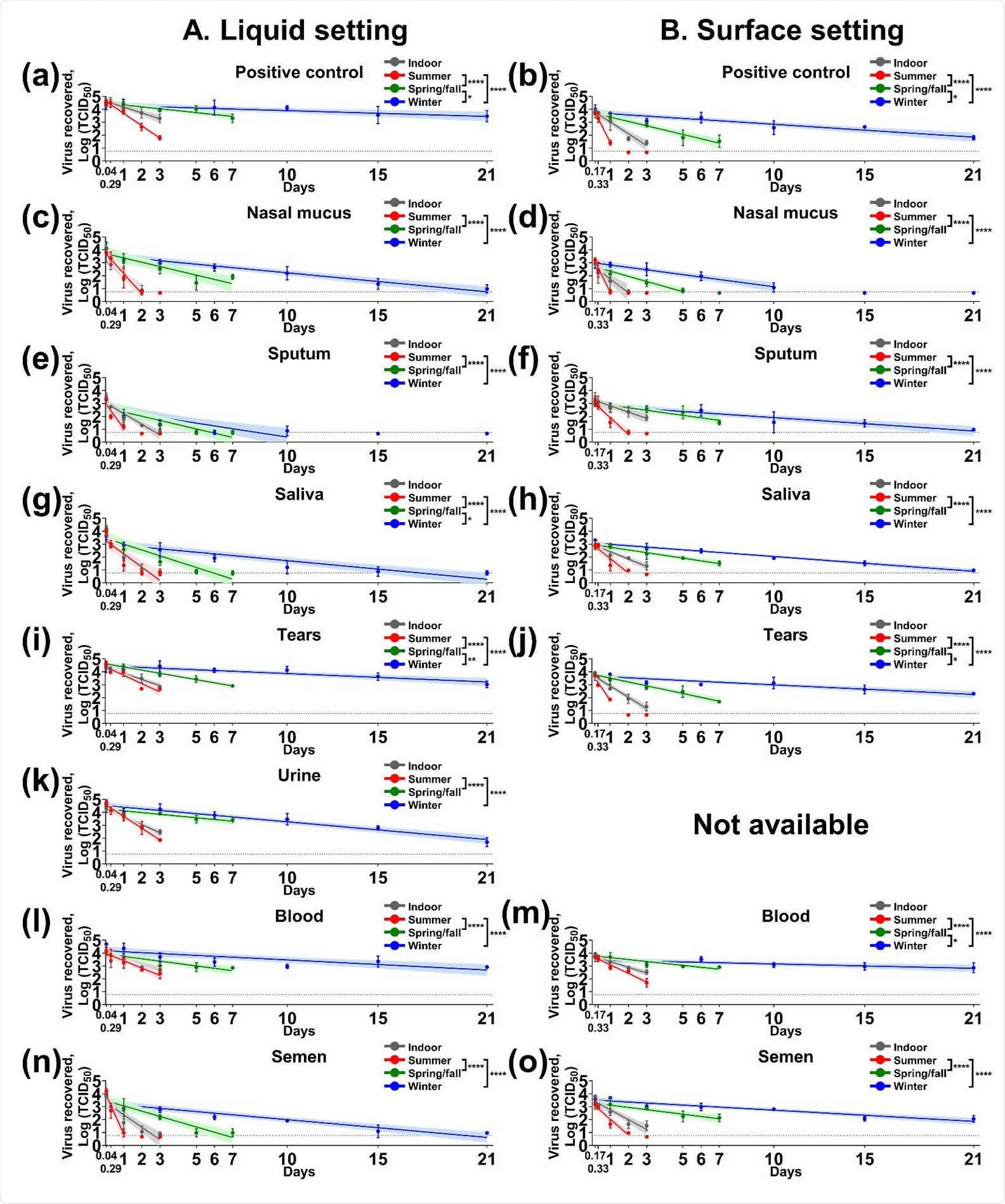
The study highlights the stability of SARS-CoV-2 in various biological fluids. The team extensively assessed the virus stability in liquid and surface settings of body floods both indoor and under different seasonal conditions.
The researchers revealed that indoors, the viral stability or half-life values (t1/2) were between 5.23 and 16.74 hours in liquid and from 6.77 to 16.57 hours on a steel surface. They also found that the virus survived between 2.3–12.57 and 2.58–10.75 hours in liquid and surface settings, respectively, under summer conditions.
During the spring or fall season, the incubation resulted in slower viral decay and longer t1/2 value, ranging from 15.98 to 54.34 hours in liquid and 18.5 to 48.4 hours on surfaces. Meanwhile, the most extended viral survival was noted under winter conditions, where the t1/2 values were between 33.37 hours and 121.83 hours in liquid and 38.55 to 235.18 hours for steel surfaces.
A previous study demonstrated that SARS-CoV-2 survived in nasal mucus and sputum for 24 hours under indoor and winter conditions. In the current study, however, the data showed that SARS-CoV-2 remained infectious for at least two days in nasal mucus for indoor and summer conditions, seven days during the spring or fall, and 21 days during the winter season.
The virus also remained stable in sputum for three days indoors, two days during the summer, seven days during the spring and fall seasons, and 21 days during the cold season. In saliva, the virus survived for a similar period.
The current study also showed the effect of temperature and relative humidity on virus survival and found that viral stability was extended during the spring/fall and winter conditions, while not during the summer.
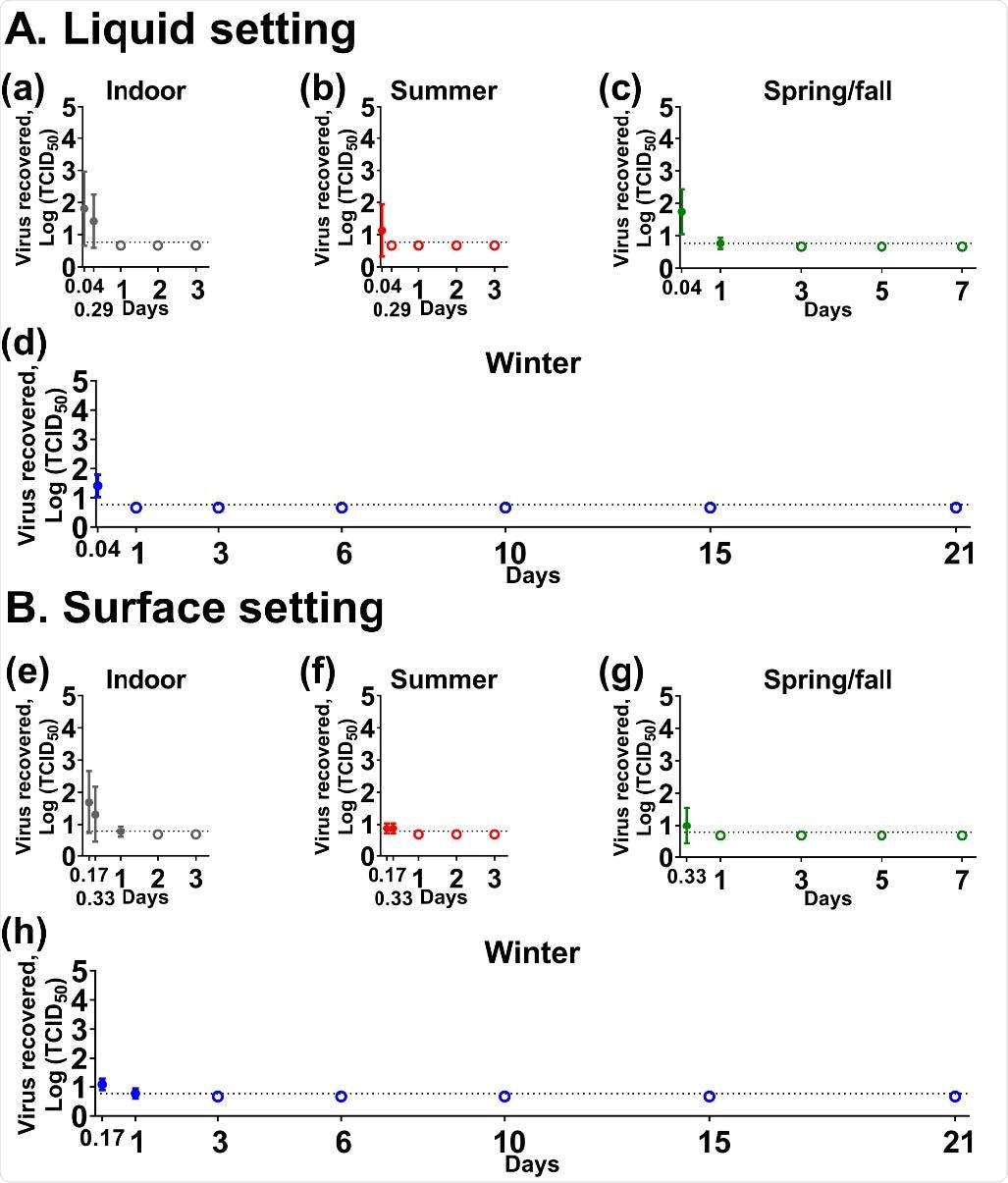
The study findings also revealed that SARS-CoV-2 is unstable in human feces and breast milk, with the infectious virus detected only up to 24 hours after contamination.
“Our findings provide new insights into the potential role of biological fluids in SARS-CoV-2 transmission and contribute to the development of public health strategies to mitigate the risk of fomites in SARS-CoV-2 transmission,” the researchers noted in the study.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) – https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- Kwon, T., Gaudreault, N., and Richt, J. (2021). Seasonal stability of SARS-CoV-2 in biological fluids. bioRxiv. https://www.biorxiv.org/content/10.1101/2021.04.07.438866v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Assay, Blood, Body Fluids, Breast Milk, Cold, Contamination, Coronavirus, Coronavirus Disease COVID-19, Cough, Public Health, Respiratory, SARS, SARS-CoV-2, Semen, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Syringe, Tissue Culture, Virus

Written by
Angela Betsaida B. Laguipo
Angela is a nurse by profession and a writer by heart. She graduated with honors (Cum Laude) for her Bachelor of Nursing degree at the University of Baguio, Philippines. She is currently completing her Master's Degree where she specialized in Maternal and Child Nursing and worked as a clinical instructor and educator in the School of Nursing at the University of Baguio.
Source: Read Full Article