Structure of central inflammation switch elucidated

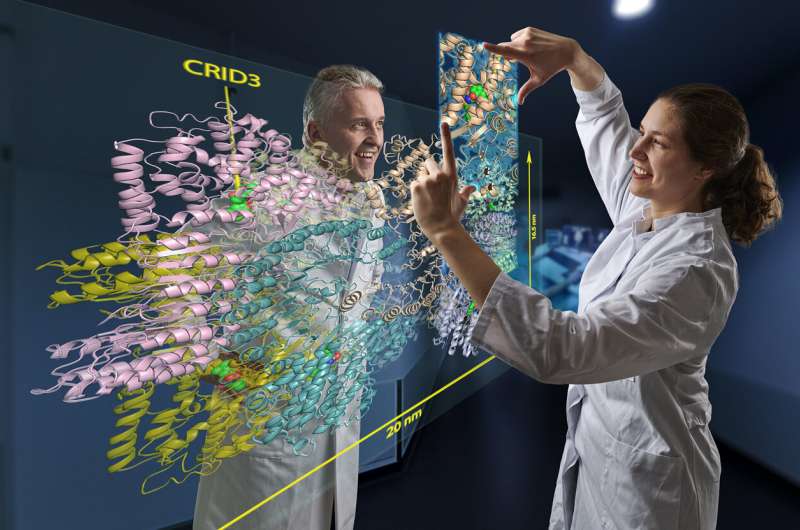
Researchers at the Universities of Bonn and Regensburg have elucidated the structure of a central cellular inflammatory switch. Their work shows which site of the giant protein called NLRP3 inhibitors can bind to. This opens the way to develop new pharmaceuticals that could target inflammatory diseases such as gout, type 2 diabetes or even Alzheimer’s disease. The results are published in the journal Nature.
In their study, the researchers investigated a protein molecule with the cryptic abbreviation NLRP3. This is a kind of danger sensor in the cell: It sounds the alarm when the cell is under stress, such as from a bacterial infection or toxins.
NLRP3 then induces the formation of pores within the cellular membrane, which ultimately results in the cell’s death. Before that, however, the sensor molecule stimulates the formation of inflammatory messenger substances that are released through the perforated membrane. These so-called cytokines recruit more immune cells to the site and ensure that cells in the surrounding area commit suicide—thereby preventing a bacterium or virus from further spreading.
“The result is a massive inflammatory response,” explains study leader Prof. Dr. Matthias Geyer from the Institute of Structural Biology at the University of Bonn. “This is certainly very useful for the defense against pathogens. But if this response is overdosed or triggered by even harmless cues, it can lead to chronic inflammatory diseases—such as type II diabetes, gout, Crohn’s disease, or even dementias like Alzheimer’s.”
Targeted containment of inflammation
Researchers around the globe are therefore seeking ways to target inflammatory processes without disrupting the entire mechanism of the immune response. As early as 20 years ago, the US pharmaceutical company Pfizer published an interesting finding in this regard: Certain active substances prevent the release of cytokines, the most important inflammatory messengers. How these CRIDs (Cytokine Release Inhibitory Drugs) do this, however, was unknown until now.
It has been known for several years that CRIDs somehow prevent cellular danger sensors from sounding the alarm. “We have now discovered the way in which they exert this effect,” explains Geyer’s colleague Inga Hochheiser. This involved isolating large amounts of NLRP3 from cells, purifying it, and adding the inhibitor CRID3. Hochheiser dropped minute portions of this mixture onto a carrier and then froze them rapidly.
This method creates a thin film of ice containing millions of NLRP3 molecules to which CRID3 is bound. These can be observed with an electron microscope. Since the molecules fall differently as they drop, different sides of them can be seen under the microscope. “These views can be combined to create a three-dimensional image,” Hochheiser explains.
The cryo-EM images allow a detailed insight into the structure of the hazard sensor inactivated by CRID3. They reveal that NLRP3 in its inactive form assembles into a mega-molecule. It consists of ten NLRP3 units that together form a kind of cage. “The most exciting result of our work, however, is that we were able to identify the CRID3 molecule docked into its binding site,” Geyer is pleased to report. “That was a tough nut that many groups worldwide have been trying to crack.”
Inhibitor prevents the activation of the giant molecule
The binding sites (structural biologists also speak of “pockets”) are located inside the cage. Each of the ten NLRP3 units has one of these pockets. When occupied by CRID3, the inhibitor blocks a flap mechanism required for NLRP3 activation. Similar to a blooming rose, which can only be visited by a bee in this state, certain parts of the NLRP3 protein reach the surface of the cage when the flap is turned over and thus become accessible.
NLRP3 is a representative of an entire family of similar proteins. Each of them presumably performs its very specific task in different inflammatory processes. “Based on our research, we believe that the pockets of all these NLRPs are different,” Geyer says. “A specific inhibitor can therefore probably be found for each of them.” This gives researchers a whole arsenal of potential new weapons against diverse, inflammatory diseases.
Source: Read Full Article