Advancing the Diagnosis and Treatment of Ovarian Cancer

 insights from industryDr. Marcos Quintela VasquezResearch and Development Innovation OfficerRBGO Research Group
insights from industryDr. Marcos Quintela VasquezResearch and Development Innovation OfficerRBGO Research GroupIn this interview, News-Medical talks to Dr. Marcos Quintela Vasquez about the work he is conducting regarding the diagnosis and treatment of ovarian cancer in partnership with CEAT and Porvair Sciences.
Please tell us about CEAT, your project aims and how the group of partners was assembled?
The Cluster for Epigenomic and Antibody Drug Conjugate (ADC) Therapeutics (CEAT) is a Swansea University-led partnership between key multi-national players Glaxo Smith Kline (GSK), Cytiva and Bruker, together with UK-based partners Porvair Sciences Ltd, Axis Bio, and BiVictriX. The CEAT project, jointly funded by the Welsh European Funding Office and collaborating partners, is designed to enable partners to deliver advances in epigenetic and ADC-based ovarian cancer therapeutics.
It is a great example of government-led initiatives to bring world-class industrial expertise alongside academic institutions to drive innovative science and sector growth.
Swansea University and the RBGO team have had successful collaborative relationships with each partner and the next step were to bring them together into a focused cluster (CEAT).

CEAT Project Team. Image Credit: CEAT
How widespread is the occurrence of ovarian cancer and how effective are current treatments?
Ovarian cancer is one of the five leading causes of cancer-related mortality in women worldwide and accounts for more deaths than any other cancer of the female reproductive system.
At present, main treatment strategies are reduced to surgery and chemotherapy, whereas targeted therapies are only aimed at a small percentage of ovarian cancer subtypes (e.g. Olaparib).
Despite enormous efforts from the research and clinical communities, the 5-year survival rate of ovarian cancer is still very low (30-40%) compared with other common malignancies such as breast cancer (80-90%).
Porvair Sciences’ experience in developing high quality products based on their bead-free Chromatrap® platform has allowed the team to successfully integrate chromatin immunoprecipitation (ChIP) into our drug development programme.”
What have been the main developments/advances made in the CEAT project thus far?
To date, the CEAT project has made significant progress in two areas of research towards treatment and diagnosis of ovarian cancer.
The first area of research is looking at the use of epigenetic drugs to target ovarian cancer cells. Epigenetics involves chemical changes to the DNA and associated proteins that can lead to genes being turned on or off.
Through CEAT, we have identified a number of epigenetic compounds of interest and are in the process of assessing (a) their clinical suitability and (b) use in deriving novel targets for treatment and companion diagnostic approaches suited to personalized medicine.
The second area of research in CEAT centers on the development of targeted therapeutics and Antibody Drug Conjugates (ADCs). ADCs are a powerful new class of therapeutics in medical oncology, where antibodies are coupled with cytotoxic agents and specifically target specific cancer cells. CEAT project partners have identified several potential ADC targets within ovarian cancer cells and are in the process of developing ADCs against these novel targets.
Image Credit: Porvair Sciences Limited
What is Porvair Sciences role in the CEAT project?
Porvair Sciences plays a primary role within the epigenetics side of the project. Porvair Sciences’ experience in developing high-quality products based on their bead-free Chromatrap® platform has allowed the team to successfully integrate chromatin immunoprecipitation (ChIP) into our drug development programme.
Specifically, Porvair Sciences’ commitment to CEAT has significantly strengthened our capabilities to investigate drug mechanisms of action to successfully investigate DNA binding events in small and/or low abundant biological samples- 3D spheroids.
We are also integrating their broader filtration products and protocols (RNA/DNA) into our investigations – enabling more selective and sensitive sample processing, yielding downstream enhancements in our genomics and epigenomics assays and approaches.
How does Porvair’s Chromatrap® ChIP technology work and what are its principal advantages?
Porvair’s Chromatrap® technology provides an agile and straightforward workflow to perform ChIP experiments.
For this purpose, Chromatrap® uses a unique patented filter-based system to selectively enrich DNA-binding proteins, significantly increasing the sensitivity of ChIP assays and minimizes routine IP-associated background noise. As opposed to typical bead-based systems, Chromatrap® kits are also easier and faster to implement, as most steps are carried out by short centrifugation steps (approx. 5h from chromatin extraction to DNA elution) and their stable 96 well-based assays are an excellent platform to expedite ChIP optimization and high throughput analysis.
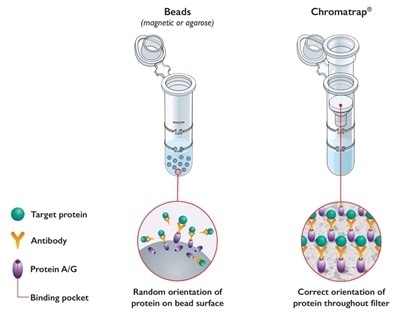
Chromatrap® Innovative Bead-free Technology. Image Credit: Porvair Sciences Limited
What new possibilities do the Chromatrap® ChIP kits for 3D spheroid analysis developed by Porvair for the CEAT project offer?
Three-dimensional (3D) culture systems have gained increased interest over the last few years as a result of their visible advantages in providing physiologically relevant information as well as accurate predictive data for in vivo clinical testing.
A great deal of clinical trial failure in the last decade has been attributed to a lack of consistency between results observed in in vitro 2D cultures and in vivo settings.
The Chromatrap® ChIP kit for 3D spheroids provides the CEAT project with a new-to-market cutting-edge tool to study the mechanism of action of epigenetic drugs in a culture system that represents more accurately the actual environment in which cancer cells reside in affected tissues.
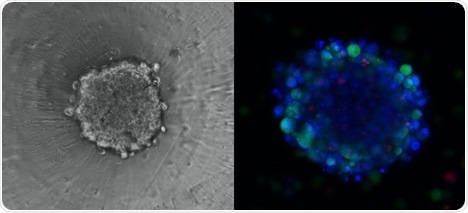
3D Spheroid with Immunostaining. Image Credit: CEAT Project/Swansea University
What are the next steps in the CEAT project and in what timeframe do you foresee realization of your target improvements in the diagnosis and treatment of ovarian cancer?
We are currently in the later stages of characterizing the mechanism of action of the lead compounds we identified to have a stronger effect on ovarian cancer cells.
We have high hopes that the impact of our upcoming publications describing the effects of such drugs will draw significant attention towards the development of potential treatment strategies in the next few years.
Meanwhile, we are working on parallel efforts to stratify ovarian cancer subtypes and disease stages using epigenomic patterns, highlighting functional genomic opportunities to better develop disease and patient management strategies.
These tools and approaches may yield new routes to market and translational benefit from our investigation pipelines. Future collaborations will be focused on the study of resistance mechanisms, the improvement of drug delivery, and combinatorial treatment strategies.
…we are working on parallel efforts to stratify ovarian cancer sub types and disease stages using epigenomic patterns, highlighting functional genomic opportunities to better develop disease and patient management strategies.”
What have you found are the principle advantages of collaborative effort to help solve big healthcare issues such as ovarian cancer?
Working collaboratively with academic, healthcare, and industrial partners have been key to CEAT’s success so far. The principal advantage of collaborating in this manner is the wealth and depth of access to a range of different expertise and being able to combine these to solve the problems at hand. CEAT brings together a variety of partners all of whom are working towards addressing an urgent need to bring new therapeutics through the early pre-clinical developmental pipeline.
Yet, each partner plays a separate role in making this happen; GSK and Swansea University focus on identifying new therapeutics, Porvair Sciences, Bruker and Cytiva are key to delivering technological solutions to enable the identification of these therapeutics, whilst Axis Bio and BiVictriX provide services that allow progression of novel therapeutics along the clinical pipeline.
Meanwhile, the NHS provides essential access to patient samples required for the research as well as a clinical perspective on the current need of patient cohorts.
Individual CEAT partners would not have the capabilities to answer the wide scope of these important research questions, however, combined we are able to deliver big advances in a relatively short space of time.
Where can readers find more information?
To find out more about our research group and project including CEAT you can visit our website at https://sites.google.com/view/rbgo/home and follow us on Twitter @RBGO_SwanseaUni. For more information on Chromatrap and Porvair Sciences, visit www.porvairsciences.com.
About the researcher

Dr. Marcos Quintela Vazquez is currently based at the Swansea University Medical School where he works as a Research and Development Innovation Officer in the Reproductive Biology and Gynaecological Oncology (RBGO) research group. He holds a Ph.D. in Nanomedicine from Swansea University, which he undertook in partnership with the Houston Methodist Research Institute in Houston, Texas (US). Dr. Quintela Vazquez’s areas of expertise include cell and molecular biology, epigenomics, bioinformatics, and drug development.
About Porvair Sciences Limited
Porvair Sciences, specialists in the manufacture of microplate products, serve Life Sciences, Biotechnology, R&D, and Molecular Biology with microplate solutions for all applications, from sample preparation to high throughput screening via our global distributor network.
Our range includes vacuum manifolds, sealers, evaporators and microtiter plates in all popular formats; deep well and shallow well storage plates, assay plates, luciferase reporter gene plates and liquid handling reagent reservoirs. We also provide custom microplate products for life science research.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Source: Read Full Article