Decoding a direct dialog between the gut microbiota and the brain

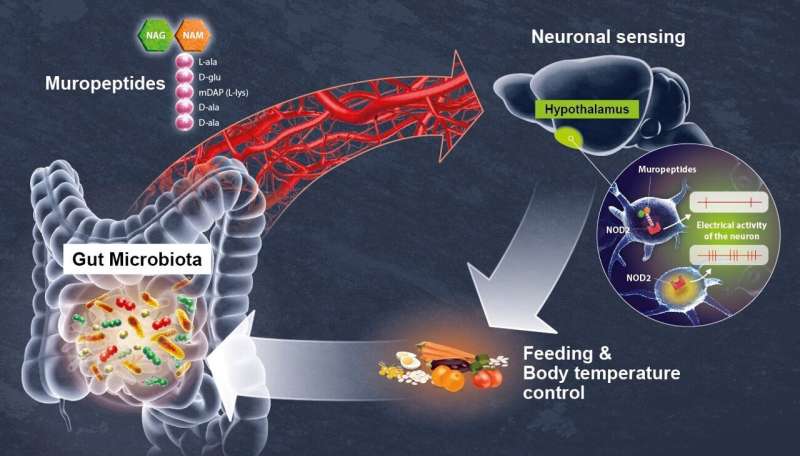
Gut microbiota by-products circulate in the bloodstream, regulating host physiological processes including immunity, metabolism and brain functions. Scientists from the Institut Pasteur (a partner research organization of Université Paris Cité), Inserm and the CNRS have discovered that hypothalamic neurons in an animal model directly detect variations in bacterial activity and adapt appetite and body temperature accordingly. These findings demonstrate that a direct dialog occurs between the gut microbiota and the brain, a discovery that could lead to new therapeutic approaches for tackling metabolic disorders such as diabetes and obesity. The findings are published in Science.
The gut is the body’s largest reservoir of bacteria. A growing body of evidence reveals the degree of interdependence between hosts and their gut microbiota, and emphasizes the importance of the gut-brain axis. At the Institut Pasteur, neurobiologists from the Perception and Memory Unit (Institut Pasteur/CNRS), immunobiologists from the Microenvironment and Immunity Unit (Institut Pasteur/Inserm), and microbiologists from the Biology and Genetics of the Bacterial Cell Wall Unit (Institut Pasteur/CNRS/Inserm) have shared their expertise to investigate how bacteria in the gut directly control the activity of particular neurons in the brain.
The scientists focused on the NOD2 (nucleotide oligomerization domain) receptor which is found inside of mostly immune cells. This receptor detects the presence of muropeptides, which are the building blocks of the bacterial cell wall. Moreover, it has previously been established that variants of the gene coding for the NOD2 receptor are associated with digestive disorders, including Crohn’s disease, as well as neurological diseases and mood disorders. However, these data were insufficient to demonstrate a direct relationship between neuronal activity in the brain and bacterial activity in the gut. This was revealed by the consortium of scientists in the new study.
Using brain imaging techniques, the scientists initially observed that the NOD2 receptor in mice is expressed by neurons in different regions of the brain, and in particular, in a region known as the hypothalamus. They subsequently discovered that these neurons’ electrical activity is suppressed when they come into contact with bacterial muropeptides from the gut. “Muropeptides in the gut, blood and brain are considered to be markers of bacterial proliferation,” explains Ivo G. Boneca, Head of the Biology and Genetics of the Bacterial Cell Wall Unit at the Institut Pasteur (CNRS/Inserm). Conversely, if the NOD2 receptor is absent, these neurons are no longer suppressed by muropeptides. Consequently, the brain loses control of food intake and body temperature. The mice gain weight and are more susceptible to developing type 2 diabetes, particularly in older females.
In this study, the scientists have demonstrated the astonishing fact that neurons perceive bacterial muropeptides directly, while this task was thought to be primarily assigned to immune cells. “It is extraordinary to discover that bacterial fragments act directly on a brain center as strategic as the hypothalamus, which is known to manage vital functions such as body temperature, reproduction, hunger and thirst,” comments Pierre-Marie Lledo, CNRS scientist and Head of the Institut Pasteur’s Perception and Memory Unit.
The neurons thus appear to detect bacterial activity (proliferation and death) as a direct gauge of the impact of food intake on the intestinal ecosystem. “Excessive intake of a specific food may stimulate the disproportionate growth of certain bacteria or pathogens, thus jeopardizing intestinal balance,” says Gérard Eberl, Head of the Microenvironment and Immunity Unit at the Institut Pasteur (Inserm).
Source: Read Full Article