SARS‑CoV‑2 Omicron variant escapes monoclonal antibodies

In a recent study published on the bioRxiv* preprint server, researchers determine the neutralizing activity of four monoclonal antibodies (mAbs) against ten strains of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that have been isolated throughout the pandemic.
Study: Omicron variant escapes therapeutic mAbs contrary to eight prior main VOC. Image Credit: ustas7777777 / Shutterstock.com
Background
Currently, anti-SARS-CoV-2 mAbs are used to induce active immunity against coronavirus disease 2019 (COVID-19) in immunocompromised patients who were unresponsive to the complete vaccine regimen. Studies have shown that different strains of SARS-CoV-2 have variations in susceptibility towards mAbs. Moreover, detailed information regarding mAb-associated neutralization of the SARS-CoV-2 Omicron variant is not yet available.
About the study
In the present study, researchers tested the neutralizing activity of mAbs such as bamlanivimab, etesevimab, casirivimab, and imdevimab against SARS-CoV-2 wild-type (B.1.1), B.1.160, Iota (B.1.526), Mu (B.1.621), Alpha (B.1.1.7), Beta (B.1.351.2), original Delta (AY.71), Delta sublineage (AY.4.2), Epsilon (B.1.429), and Omicron (B.1.1.529) strains.
Vero E6 cells were cultured without antibiotics in a minimum essential medium (MEM) and then used for the neutralization tests of SARS-CoV-2 in MEM growth medium with fetal bovine serum (FBS) and glutamine.
The ten SARS-CoV-2 strains used in this study were isolated from SARS-CoV-2-positive nasopharyngeal swabs in cell culture and stored at -80°C in IHU Méditerranée Infection institute. Later, the supernatant of each strain was harvested and genotyped using whole-genome next-generation (NGS) sequencing technology.
Neutralization tests were carried out by inoculating the viral strains into Vero E6 cells. Two days after viral infection, the suspension was quantified using reverse transcription-polymerase chain reaction (RT-PCR) and median tissue culture infectious dose (TCID50) assay.
All mAbs used in the study including etesevimab, bamlavinimab, imdevimab, and casirivimab were diluted in 1:5 serial dilutions. For the combination of etesevimab + bamlavinimab and casirivimab + imdevimab, the highest concentration of the mixtures was tested against two times more etesevimab than bamlavinimab and two times more casirivimab than imdevimab mixtures, respectively.
A microneutralization assay was conducted by mixing mAb dilutions with each viral strain. The mAb titer required to obtain 50% neutralization against SARS-CoV-2 variants was determined using an inverted optical microscope five days after viral infection. Moreover, mAbs and their combinations were tested three times against all SARS-CoV-2 variants, except Omicron, which was tested four times.
Study findings
Bamlavinimab did not inhibit the SARS-CoV-2 Mu, Epsilon, Delta, and Beta variants. Etesevimab showed 50% neutralization below 5 µg/mL of the SARS-CoV-2 Epsilon, wild-type, and both Delta variants. In etesevimab and bamlanivimab combinations, significant neutralization was detected against the B.1.160, Iota, and Alpha strains.
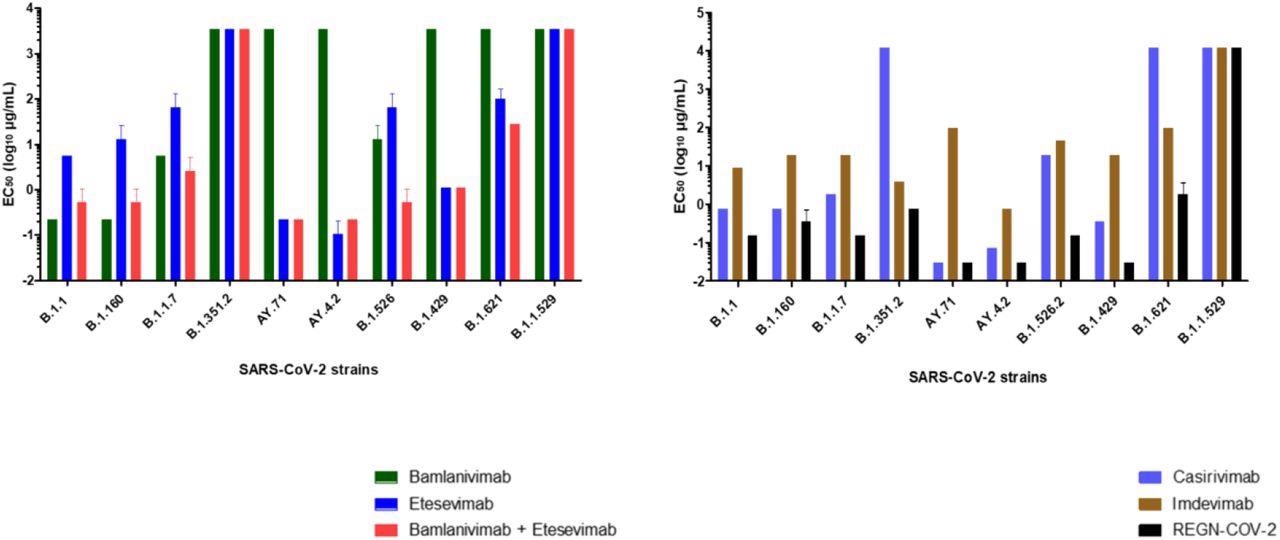
Concentrations required obtaining 50% neutralization (EC50 log10 µg/mL) for each mAb. (A) bamlanivimab, etesevimab, mixture of bamlanivimab and etesevimab, (B) casirivimab, imdevimab and REGN-CoV-2 on the 10 SARS-CoV-2 strains tested. Each mAb was tested three times (except for Omicron variant 4 times).
Casirivimab showed remarkable neutralization against the B.1.160, wild-type, Alpha, Iota, Epsilon, and both Delta variants of SARS-CoV-2. In contrast, casirivimab did not neutralize SARS-CoV-2 Beta and Mu variants.
Imdevimab neutralized all SARS-CoV-2 variants except Omicron. However, imdevimab concentrations required for 50% neutralization of SARS-CoV-2 variants were more than that of casirivimab.
The casirivimab + imdevimab combination showed a synergistic effect, especially against the Epsilon, AY4.2, and AY.71 variants, with 50% neutralization at 0.03 µg/mL concentration. The casirivimab + imdevimab combination showed 50% neutralization at 0.2, 0.4, and 0.7 µg/mL concentrations against the SARS-CoV-2 Original, Iota, Alpha, and B.1.160, and Beta variants, respectively.
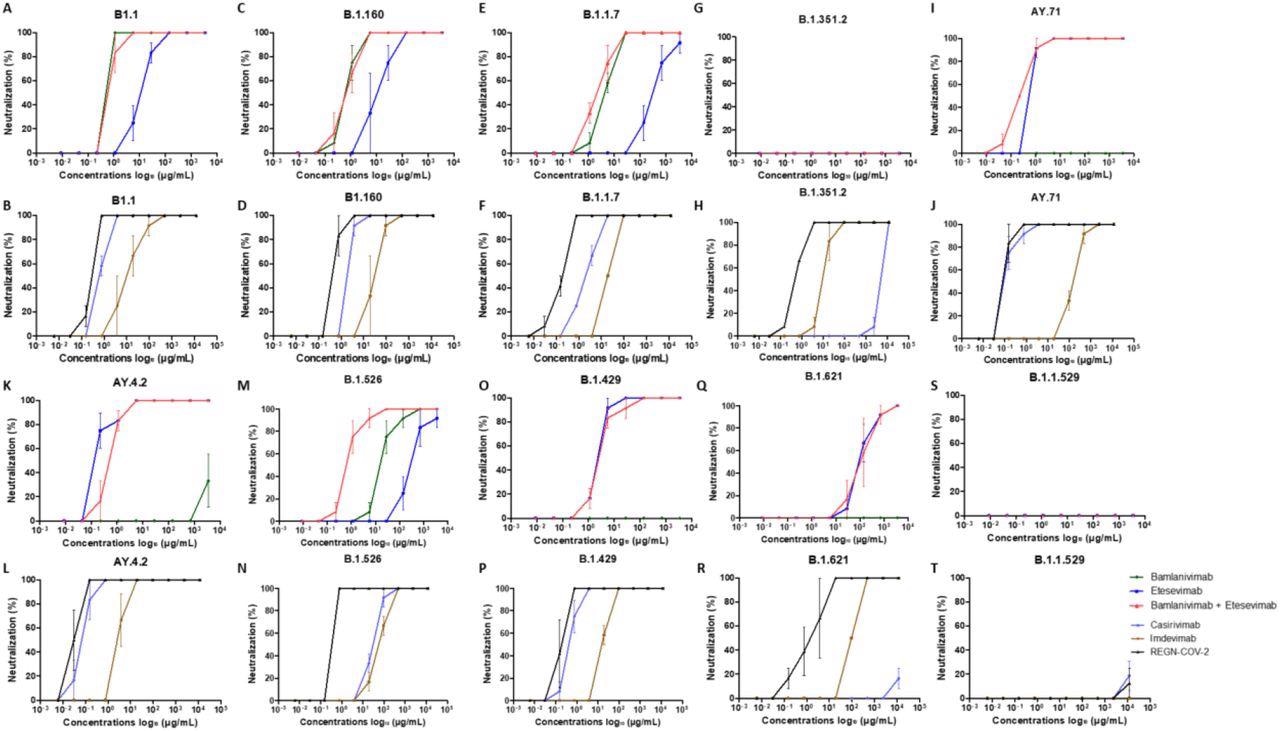
Neutralization curves in Vero E6 cells for each strains tested with each mAb : A, C, E, G, I, K, M, N, O, Q, S : bamlanivimab, etesevimab and mixture of bamlanivimab and etesevimab – B, D, F, H, J, L, N, P, R, T : casirivimab, imdevimab and REGN-CoV-2. Each experiment was done three times, except for the Omicron variant four times.
At a concentration of 2 µg/mL, the casirivimab + imdevimab combination showed 50% neutralization against the SARS-CoV-2 Mu variant. However, none of the four mAbs alone or in combination exhibited neutralizing capacity against Omicron.
Conclusions
The study findings suggest that four mAbs tested had a lower inhibitory effect on recently emerging SARS-CoV-2 variants. However, their combination was highly effective, particularly against the Delta variant. In contrast, the four mAbs combined or alone were ineffective against the Omicron variant.
The present study underscores the reinforcement of protective measures against SARS-CoV-2 Omicron infection among immunocompromised patients. However, further studies are required to confirm the ineffectiveness of mAbs against the Omicron variant.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Boschi, C., Colson, P., Bancod, A., et al. (2021). Omicron variant escapes therapeutic mAbs contrary to eight prior main VOC. bioRxiv. doi:10.1101/2022.01.03.474769. https://www.biorxiv.org/content/10.1101/2022.01.03.474769v1.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Assay, Cell, Cell Culture, Coronavirus, Coronavirus Disease COVID-19, covid-19, Genome, Glutamine, immunity, Microscope, Nasopharyngeal, Omicron, Pandemic, Polymerase, Polymerase Chain Reaction, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Tissue Culture, Transcription, Vaccine

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article